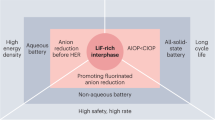
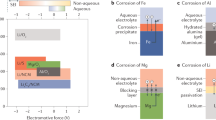
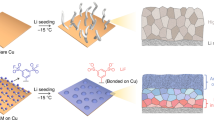
Abstract
The future of electrochemical energy storage hinges on the advancement of science and technology that enables rechargeable batteries that utilize reactive metals as anodes. With specific capacity more than ten times that of the LiC6 anode used in present-day lithium-ion batteries, cells based on Li-metal anodes are of particular interest. Effective strategies for stabilizing the anode in such cells are now understood to be a requirement for progress on exceptional storage technologies, including Li–S and Li–O2 batteries. Multiple challenges—parasitic reactions of Li-metal with liquid electrolytes, unstable and dendritic electrodeposition, and dendrite-induced short circuits—derailed early efforts to commercialize such lithium-metal batteries. Here we consider approaches for rationally designing electrolytes and Li-metal/electrolyte interfaces for stable, dendrite-free operation of lithium-metal batteries. On the basis of fundamental understanding of the failure modes of reactive metal anodes, we discuss the key variables that govern the stability of electrodeposition at the Li anode and propose a universal framework for designing stable electrolytes and interfaces for lithium-metal batteries.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Whittingham, M. S. Electrical energy storage and intercalation chemistry. Science 192, 1126–1127 (1976).
Fleury, V., Chazalviel, J.-N. & Rosso, M. Coupling of drift, diffusion, and electroconvection, in the vicinity of growing electrodeposits. Phys. Rev. E 48, 1279–1295 (1993).
Aurbach, D., Zinigrad, E., Cohen, Y. & Teller, H. A short review of failure mechanisms of lithium metal and lithiated graphite anodes in liquid electrolyte solutions. Solid State Ionics 148, 405–416 (2002).
Sawada, Y., Dougherty, A. & Gollub, J. P. Dendritic and fractal patterns in electrolytic metal deposits. Phys. Rev. Lett. 56, 1260–1263 (1986).
Rosso, M., Chazalviel, J-N. & Chassaing, E. Calculation of the space charge in electrodeposition from a binary electrolyte. J. Electroanal. Chem. 587, 323–328 (2006).
Lu, Y., Korf, K. S., Kambe, Y., Tu, Z. & Archer, L. A. Ionic-liquid–nanoparticle hybrid electrolytes: applications in lithium metal batteries. Angew. Chem. Int. Ed. 53, 488–492 (2014).
Aogaki, R. & Makino, T. Theory of powdered metal formation in electrochemistry — morphological instability in galvanostatic crystal growth under diffusion control. Electrochim. Acta 26, 1509–1517 (1981).
Tikekar, M. D., Archer, L. A. & Koch, D. L. Stability analysis of electrodeposition across a structured electrolyte with immobilized anions. J. Electrochem. Soc. 161, A847–A855 (2014).
Monroe, C. & Newman, J. The impact of elastic deformation on deposition kinetics at lithium/polymer interfaces. J. Electrochem. Soc. 152, A396–A404 (2005).
Stone, G. M. et al. Resolution of the modulus versus adhesion dilemma in solid polymer electrolytes for lithium metal batteries. J. Electrochem. Soc. 159, A222–A227 (2012).
Ozhabes, Y., Gunceler, D. & Arias, T. A. Stability and surface diffusion at lithium-electrolyte interphases with connections to dendrite suppression. Preprint at http://arxiv.org/abs/1504.05799 (2015).
Tikekar, M. D., Archer, L. A. & Koch, D. L. Stabilizing electrodeposition in elastic solid electrolytes containing immobilized anions. Sci. Adv. 2, E1600320 (2016).
Tu, Z., Nath, P., Lu, Y., Tikekar, M. D. & Archer, L. A. Nanostructured electrolytes for stable lithium electrodeposition in secondary batteries. Acc. Chem. Res. 48, 2947–2956 (2015).
Bates, J. B. et al. Electrical properties of amorphous lithium electrolyte thin films. Solid State Ionics 53–56, 647–654 (1992).
Kanno, R. & Murayama, M. Lithium ionic conductor thio-LISICON: the Li2S–GeS2–P2S5 system. J. Electrochem. Soc. 148, 742–746 (2001).
Bates, J. B. et al. Fabrication and characterization of amorphous lithium electrolyte thin films and rechargeable thin-film batteries. J. Power Sources 43, 103–110 (1993).
De Jonghe, L. C., Feldman, L. & Millett, P. Some geometrical aspects of breakdown of sodium beta alumina. Mater. Res. Bull. 14, 589–595 (1979).
Lu, Y. et al. Stable cycling of lithium metal batteries using high transference number electrolytes. Adv. Energy Mater. 5, 1402073 (2015).
Song, J., Lee, H., Choo, M.-J., Park, J.-K. & Kim, H.-T. Ionomer-liquid electrolyte hybrid ionic conductor for high cycling stability of lithium metal electrodes. Sci. Rep. 5, 14458 (2015).
Cheng, X. B. et al. Dendrite-free lithium deposition induced by uniformly distributed lithium ions for efficient lithium metal batteries. Adv. Mater. 28, 2888–2895 (2016).
Bouchet, R. et al. Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries. Nat. Mater. 12, 452–457 (2013).
Schaefer, J. L., Yanga, D. A. & Archer, L. A. High lithium transference number electrolytes via creation of 3-dimensional, charged, nanoporous networks from dense functionalized nanoparticle composites. Chem. Mater. 25, 834–839 (2013).
Smith, D. M., Cheng, S., Wang, W., Bunning, T. J. & Li, C. Y. Polymer electrolyte membranes with exceptional conductivity anisotropy via holographic polymerization. J. Power Sources 271, 597–603 (2014).
Chen, Q., Geng, K. & Sieradzki, K. Prospects for dendrite-free cycling of Li metal batteries. J. Electrochem. Soc. 162, A2004–A2007 (2015).
Liu, Y. et al. Lithium-coated polymeric matrix as a minimum volume-change and dendrite-free lithium metal anode. Nat. Commun. 7, 10992 (2016).
Bron, P. et al. Li10SnP2S12: an affordable lithium superionic conductor. J. Am. Chem. Soc. 135, 15694–15697 (2013).
Lapp, R., Skaarup, S. & Hooper, A. Ionic conductivity of pure and doped Li3N. Solid State Ionics 11, 97–103 (1983).
Tu, Z., Kambe, Y., Lu, Y. & Archer, L. A. Nanoporous polymer-ceramic composite electrolytes for lithium metal batteries. Adv. Energy Mater. 4, 1300654 (2014).
Giles, J. R. M., Gray, F. M., Maccallum, J. R. & Vincent, C. A. Synthesis and characterization of ABA block copolymer-based polymer electrolytes. Polymer 28, 1977–1981 (1987).
Khurana, R., Schaefer, J. L., Archer, L. A. & Coates, G. W. Suppression of lithium dendrite growth using cross-linked polyethylene/poly(ethylene oxide) electrolytes: a new approach for practical lithium-metal polymer batteries. J. Am. Chem. Soc. 136, 7395–7402 (2014).
Pan, Q., Smith, D. M., Qi, H., Wang, S. & Li, C. Y. Hybrid electrolytes with controlled network structures for lithium metal batteries. Adv. Mater. 27, 5995–6001 (2015).
Choudhury, S., Mangal, R., Agrawal, A. & Archer, L. A. A highly reversible room-temperature lithium metal battery based on crosslinked hairy nanoparticles. Nat. Commun. 6, 10101 (2015).
Gurevitch, I. et al. Nanocomposites of titanium dioxide and polystyrene-poly(ethylene oxide) block copolymer as solid-state electrolytes for lithium metal batteries. J. Electrochem. Soc. 160, A1611–A1617 (2013).
Tung, S.-O., Ho, S., Yang, M., Zhang, R. & Kotov, N. A. A dendrite-suppressing composite ion conductor from aramid nanofibres. Nat. Commun. 6, 6152 (2015).
Miao, R. et al. Novel dual-salts electrolyte solution for dendrite-free lithium-metal based rechargeable batteries with high cycle reversibility. J. Power Sources 271, 291–297 (2014).
Qian, J. et. al. High rate and stable cycling of lithium metal anode. Nat. Commun. 6, 6362 (2015).
Seh, Z. W., Sun, J., Sun, Y. & Cui, Y. A highly reversible room-temperature sodium metal anode. ACS Cent. Sci. 1, 449–455 (2015).
Lu, Y., Tu, Z. & Archer, L. A. Stable lithium electrodeposition in liquid and nanoporous solid electrolytes. Nat. Mater. 13, 961–969 (2014).
Choudhury, S. & Archer, L. A. Lithium fluoride additives for stable cycling of lithium batteries at high current densities. Adv. Electron. Mater. 2, 1500246 (2016).
Ding, F. et al. Dendrite-free lithium deposition via self-healing electrostatic shield mechanism. J. Am. Chem. Soc. 135, 4450–4456 (2013).
Zheng, G. et al. Interconnected hollow carbon nanospheres for stable lithium metal anodes. Nat. Nanotech. 9, 618–623 (2014).
Kozen, A. C. et al. Next-generation lithium metal anode engineering via atomic layer deposition. ACS Nano 9, 5884–5892 (2015).
Neudecker, B. J., Dudney, N. J. & Bates, J. B. “Lithium-free” thin-film battery with in situ plated Li anode. J. Electrochem. Soc. 147, 517–523 (2000).
Sun, Y. et al. High-capacity battery cathode prelithiation to offset initial lithium loss. Nat. Energy 1, 15008 (2016).
Bhattacharyya, R. et al. In situ NMR observation of the formation of metallic lithium microstructures in lithium batteries. Nat. Mater. 9, 504–510 (2010).
Harry, K. J., Hallinan, D. T., Parkinson, D. Y., MacDowell, A. A. & Balsara, N. P. Detection of subsurface structures underneath dendrites formed on cycled lithium metal electrodes. Nat. Mater. 13, 69–73 (2014).
Williamson, M., Tromp, R., Vereecken, P., Hull, R. & Ross, F. Dynamic microscopy of nanoscale cluster growth at the solid–liquid interface. Nat. Mater. 2, 532–536 (2003).
White, E. R. et al. In situ transmission electron microscopy of lead dendrites and lead ions in aqueous solution. ACS Nano 6, 6308–6317 (2012).
Han, J.-H., Khoo, E., Bai, P. & Bazant, M. Z. Over-limiting current and control of dendritic growth by surface conduction in nanopores. Sci. Rep. 4, 7056 (2014).
Xu, S., Lu, Y., Wang, H., Abruna, H. D. & Archer, L. A. A rechargeable Na-CO2/O2 battery enabled by stable nanoparticle hybrid electrolytes. J. Mater. Chem. A 2, 17723–17729 (2014).
Al Sadat, W. I. & Archer, L. A. The O2-assisted Al/CO2 electrochemical cell: A system for CO2 capture/conversion and electric power generation. Sci. Adv. 2, e1600968 (2016).
Liu, Q.-C. et al. Artificial protection film on lithium metal anode toward long-cycle life lithium-oxygen batteries. Adv. Mater. 27, 5241–5247 (2015).
Stark, J. K., Ding, Y. & Kohl, P. A. Nucleation of electrodeposited lithium metal: dendritic growth and the effect of co-deposited sodium. J. Electrochem. Soc. 160, D337–D342 (2013).
Acknowledgements
This material is based on work supported by the National Science Foundation Award No. DMR-1609125 and by the Energy Materials Center at Cornell, an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, and Office of Basic Energy Sciences under Award Number DESC0001086.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
L.A.A. is a founder and holds a financial interest in NOHMs Technologies, a technology concern seeking to commercialize electrolytes and electrodes for high voltage Li-ion and high-energy Li's batteries.
Rights and permissions
About this article
Cite this article
Tikekar, M., Choudhury, S., Tu, Z. et al. Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat Energy 1, 16114 (2016). https://doi.org/10.1038/nenergy.2016.114
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nenergy.2016.114
This article is cited by
-
Single [0001]-oriented zinc metal anode enables sustainable zinc batteries
Nature Communications (2024)
-
Harmfulness of polysemantic terms in electrochemistry
Journal of Solid State Electrochemistry (2024)
-
Naked metallic skin for homo-epitaxial deposition in lithium metal batteries
Nature Communications (2023)
-
Homogeneous and mechanically stable solid–electrolyte interphase enabled by trioxane-modulated electrolytes for lithium metal batteries
Nature Energy (2023)
-
A jigsaw-structured artificial solid electrolyte interphase for high-voltage lithium metal batteries
Communications Materials (2023)