Abstract
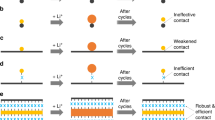
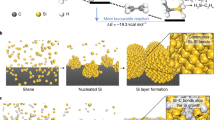
Existing anode technologies are approaching their limits, and silicon is recognized as a potential alternative due to its high specific capacity and abundance. However, to date the commercial use of silicon has not satisfied electrode calendering with limited binder content comparable to commercial graphite anodes for high energy density. Here we demonstrate the feasibility of a next-generation hybrid anode using silicon-nanolayer-embedded graphite/carbon. This architecture allows compatibility between silicon and natural graphite and addresses the issues of severe side reactions caused by structural failure of crumbled graphite dust and uncombined residue of silicon particles by conventional mechanical milling. This structure shows a high first-cycle Coulombic efficiency (92%) and a rapid increase of the Coulombic efficiency to 99.5% after only 6 cycles with a capacity retention of 96% after 100 cycles, with an industrial electrode density of >1.6 g cm−3, areal capacity loading of >3.3 mAh cm−2, and <4 wt% binding materials in a slurry. As a result, a full cell using LiCoO2 has demonstrated a higher energy density (1,043 Wh l−1) than with standard commercial graphite electrodes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Jeong, G., Kim, Y.-U., Kim, H., Kim, Y.-J. & Sohn, H.-J. Prospective materials and applications for Li secondary batteries. Energy Environ. Sci. 4, 1986–2002 (2011).
Crabtree, G., Kócs, E. & Trahey, L. The energy-storage frontier: lithium-ion batteries and beyond. MRS Bull. 40, 1067–1078 (2015).
Choi, N. S. et al. Challenges facing lithium batteries and electrical double-layer capacitors. Angew. Chem. Int. Ed. 51, 9994–10024 (2012).
Thackeray, M. M., Wolverton, C. & Isaacs, E. D. Electrical energy storage for transportation—approaching the limits of, and going beyond, lithium-ion batteries. Energy Environ. Sci. 5, 7854–7863 (2012).
Goodenough, J. B. Evolution of strategies for modern rechargeable batteries. Acc. Chem. Res. 46, 1053–1061 (2013).
Rolison, D. R. & Nazar, L. F. Electrochemical energy storage to power the 21st century. MRS Bull. 36, 486–493 (2011).
Zhang, W. J. A review of the electrochemical performance of alloy anodes for lithium-ion batteries. J. Power Sources 196, 13–24 (2011).
Obrovac, M. N. & Chevrier, V. L. Alloy negative electrodes for Li-ion batteries. Chem. Rev. 114, 11444–11502 (2014).
Nitta, N. & Yushin, G. High-capacity anode materials for lithium-ion batteries: choice of elements and structures for active particles. Part. Part. Syst. Charact. 31, 317–336 (2014).
Obrovac, M. N. & Christensen, L. Structural changes in silicon anodes during lithium insertion/extraction. Electrochem. Solid-State Lett. 7, A93–A96 (2004).
Mcdowell, M. T., Lee, S. W., Nix, W. D. & Cui, Y. 25th anniversary article: understanding the lithiation of silicon and other alloying anodes for lithium-ion batteries. Adv. Mater. 25, 4966–4985 (2013).
Ko, M., Oh, P., Chae, S., Cho, W. & Cho, J. Considering critical factors of Li-rich cathode and Si anode materials for practical Li-ion cell applications. Small 11, 4058–4073 (2015).
Wu, H. & Cui, Y. Designing nanostructured Si anodes for high energy lithium ion batteries. Nano Today 7, 414–429 (2012).
Wu, H. et al. Stable cycling of double-walled silicon nanotube battery anodes through solid-electrolyte interphase control. Nature Nanotech. 7, 309–314 (2012).
Liu, N. et al. A yolk-shell design for stabilized and scalable Li-ion battery alloy anodes. Nano Lett. 12, 3315–3321 (2012).
Lu, Z. et al. Nonfilling carbon coating of porous silicon micrometer-sized particles for high-performance lithium battery anodes. ACS Nano 9, 2540–2547 (2015).
Liu, N. et al. A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes. Nature Nanotech. 9, 187–192 (2014).
Luo, J. et al. Crumpled graphene-encapsulated Si nanoparticles for lithium ion battery anodes. J. Phys. Chem. Lett. 3, 1824–1829 (2012).
Li, Y. et al. Growth of conformal graphene cages on micrometre-sized silicon particles as stable battery anodes. Nature Energy 1, 15029 (2016).
Lee, J.-I. & Park, S. High-performance porous silicon monoxide anodes synthesized via metal-assisted chemical etching. Nano Energy 2, 146–152 (2013).
Song, J.-W., Nguyen, C. C. & Song, S.-W. Stabilized cycling performance of silicon oxide anode in ionic liquid electrolyte for rechargeable lithium batteries. RSC Adv. 2, 2003–2009 (2012).
Miyachi, M., Yamamoto, H., Kawai, H., Ohta, T. & Shirakata, M. Analysis of SiO anodes for lithium-ion batteries. J. Electrochem. Soc. 152, A2089–A2091 (2005).
Suh, S. S. et al. Electrochemical behavior of SiOx anodes with variation of oxygen ratio for Li-ion batteries. Electrochim. Acta 148, 111–117 (2014).
Dimov, N., Xia, Y. & Yoshio, M. Practical silicon-based composite anodes for lithium-ion batteries: fundamental and technological features. J. Power Sources 171, 886–893 (2007).
Du, Z., Dunlap, R. A. & Obrovac, M. N. High energy density calendered Si alloy/graphite anodes. J. Electrochem. Soc. 161, A1698–A1705 (2014).
Jo, Y. N. et al. Si–graphite composites as anode materials for lithium secondary batteries. J. Power Sources 195, 6031–6036 (2010).
Lee, J.-H., Kim, W.-J., Kim, J.-Y., Lim, S.-H. & Lee, S.-M. Spherical silicon/graphite/carbon composites as anode material for lithium-ion batteries. J. Power Sources 176, 353–358 (2008).
Li, M. et al. Facile spray-drying/pyrolysis synthesis of core–shell structure graphite/silicon-porous carbon composite as a superior anode for Li-ion batteries. J. Power Sources 248, 721–728 (2014).
Yoon, Y. S., Jee, S. H., Lee, S. H. & Nam, S. C. Nano Si-coated graphite composite anode synthesized by semi-mass production ball milling for lithium secondary batteries. Surf. Coat. Technol. 206, 553–558 (2011).
Alias, M. et al. Silicon/graphite nanocomposite electrode prepared by low pressure chemical vapor deposition. J. Power Sources 174, 900–904 (2007).
Kasavajjula, U., Wang, C. S. & Appleby, A. J. Nano- and bulk-silicon-based insertion anodes for lithium-ion secondary cells. J. Power Sources 163, 1003–1039 (2007).
Saint, J. et al. Towards a fundamental understanding of the improved electrochemical performance of silicon-carbon composites. Adv. Funct. Mater. 17, 1765–1774 (2007).
Yoshio, M. et al. Carbon-coated Si as a lithium-ion battery anode material. J. Electrochem. Soc. 149, A1598–A1603 (2002).
Yoshio, M. et al. Improvement of natural graphite as a lithium-ion battery anode material, from raw flake to carbon-coated sphere. J. Mater. Chem. 14, 1754–1758 (2004).
Uono, H., Kim, B.-C., Fuse, T., Ue, M. & Yamaki, J.-I. Optimized structure of silicon/carbon/graphite composites as an anode material for Li-ion batteries. J. Electrochem. Soc. 153, A1708–A1713 (2006).
Lee, E. H. et al. Effect of carbon matrix on electrochemical performance of Si/C composites for use in anodes of lithium secondary batteries. Bull. Korean Chem. Soc. 34, 1435–1440 (2013).
Lee, S. W. et al. Kinetics and fracture resistance of lithiated silicon nanopillar pairs controlled by their mechanical interaction. Nature Commun. 6, 7533 (2015).
Liu, X. H. et al. Ultrafast electrochemical lithiation of individual Si nanowire anodes. Nano Lett. 11, 2251–2258 (2011).
Mcdowell, M. T. et al. In situ TEM of two-phase lithiation of amorphous silicon nanospheres. Nano Lett. 13, 758–764 (2013).
Sethuraman, V. A., Chon, M. J., Shimshak, M., Srinivasan, V. & Guduru, P. R. In situ measurements of stress evolution in silicon thin films during electrochemical lithiation and delithiation. J. Power Sources 195, 5062–5066 (2010).
Boylan, J. Smooth operators: carbon-graphite materials. Mater. World 4, 707–708 (1996).
Acknowledgements
This work was supported by the IT R&D programme of the Ministry of Trade, Industry & Energy/Korea Evaluation Institute of Industrial Technology (MOTIE/KEIT) (Development of Li-rich Cathode and Carbon-free Anode Materials for High Capacity/High Rate Lithium Secondary Batteries, 10046306) and the 2016 Research Fund (1.160033.01) of UNIST (Ulsan National Institute of Science and Technology). Also, Y.C. acknowledges support from the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies, Battery Materials Research Program of the US Department of Energy.
Author information
Authors and Affiliations
Contributions
M.K. conceived and designed the experiments, and performed data interpretation. M.K. prepared the samples and carried out the main experiments. S.C. assisted with the data interpretation and designed the experimental scheme; H.-W.L. conducted in situ TEM characterization and detailed discussion on analytic results. J.M. assisted with sample preparation and performed the energy-dispersive spectroscopy measurement. N.K. provided data analysis. M.K., S.C., H.-W.L., Y.C. and J.C. co-wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1–9, Supplementary Tables 1 and 2, Supplementary Notes, Supplementary References. (PDF 938 kb)
Supplementary Video 1
Lithiation of a Si nanolayer coated on the surface of empty volume space. The Si nanolayer can expand toward empty volume space upon lithiation, leading to no rupture and interference between Si and graphite. The movie is played at 10× speed. (MP4 16592 kb)
Supplementary Video 2
Lithiation of a Si nanolayer located in the gap between graphite layers. The Si nanolayer located in the gap between the graphite layers remains intact without any cracks and contact losses upon lithiation. The movie is played at 7× speed. (MP4 11800 kb)
Rights and permissions
About this article
Cite this article
Ko, M., Chae, S., Ma, J. et al. Scalable synthesis of silicon-nanolayer-embedded graphite for high-energy lithium-ion batteries. Nat Energy 1, 16113 (2016). https://doi.org/10.1038/nenergy.2016.113
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nenergy.2016.113
This article is cited by
-
Electrode Conditions of Lithium-Ion Cell for Achieving High Energy Density
Korean Journal of Chemical Engineering (2024)
-
ZnFe2O4/Graphite Composite with High Performance as Anode Material for Lithium-Ion Batteries
Electronic Materials Letters (2023)
-
Origin of enhanced stability of SiO anode via using carbon nanotubes
Science China Materials (2023)
-
Intertwining porous silicon with conducting polymer for high-efficiency stable Li-ion battery anodes
Korean Journal of Chemical Engineering (2023)
-
Three-dimensional hierarchically porous MoS2 foam as high-rate and stable lithium-ion battery anode
Nature Communications (2022)