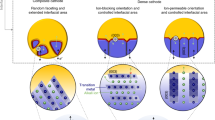
Abstract
The critical region determining the performance and lifetime of solid oxide electrochemical systems is normally at the electrode side of the electrode/electrolyte interface. Typically this electrochemically active region only extends a few micrometres and for best performance involves intricate structures and nanocomposites. Much of the most exciting recent research involves understanding processes occurring at this interface and in developing new means of controlling the structure at this interface on the nanoscale. Here we consider in detail the diverse range of materials architectures that may be involved, describe the evolution of these interface structures and finally explore the new chemistries that allow control and manipulation of these architectures to optimize both performance and durability.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Atkinson, A. et al. Advanced anodes for high-temperature fuel cells. Nature Mater. 3, 17–27 (2004).
Gauckler, L. J. et al. Solid oxide fuel cells: systems and materials. CHIMIA Int. J. Chem. 58, 837–850 (2004).
McIntosh, S. & Gorte, R. J. Direct hydrocarbon solid oxide fuel cells. Chem. Rev. 104, 4845–4866 (2004).
Gorte, R. J. & Vohs, J. M. Nanostructured anodes for solid oxide fuel cells. Curr. Opin. Colloid Interface Sci. 14, 236–244 (2009).
Cowin, P. I., Petit, C. T. G., Lan, R., Irvine, J. T. S. & Tao, S. Recent progress in the development of anode materials for solid oxide fuel cells. Adv. Energy Mater. 1, 314–332 (2011).
Irvine, J. T. S., Sinclair, D. C. & West, A. R. Electroceramics: characterization by impedance spectroscopy. Adv. Mater. 2, 132–138 (1990).
Adler, S. B. Limitations of charge-transfer models for mixed-conducting oxygen electrodes. Solid State Ion. 135, 603–612 (2000).
Adler, S. B. & Bessler, W. G. in Handbook of Fuel Cells 441–463 (Wiley, 2010); http://go.nature.com/IM8hMk
Ebbesen, S. D., Jensen, S. H., Hauch, A. & Mogensen, M. B. High temperature electrolysis in alkaline cells, solid proton conducting cells, and solid oxide cells. Chem. Rev. 114, 10697–10734 (2014).
Wilson, J. R. et al. Three-dimensional reconstruction of a solid-oxide fuel-cell anode. Nature Mater. 5, 541–544 (2006).
Izzo, J. R. et al. Nondestructive reconstruction and analysis of SOFC anodes using X-ray computed tomography at sub-50 nm resolution. J. Electrochem. Soc. 155, B504–B508 (2008).
Liu, J., Hull, S., Ahmed, I. & Skinner, S. J. Application of combined neutron diffraction and impedance spectroscopy for in-situ structure and conductivity studies of La2Mo2O9 . Nucl. Instrum. Methods Phys. Res. Sec. B 269, 539–543 (2011).
Woolley, R. J., Ryan, M. P. & Skinner, S. J. In situ measurements on solid oxide fuel cell cathodes – simultaneous X-ray absorption and AC impedance spectroscopy on symmetrical cells. Fuel Cells 13, 1080–1087 (2013).
Kubicek, M., Limbeck, A., Frö mling, T., Hutter, H. & Fleig, J. Relationship between cation segregation and the electrochemical oxygen reduction kinetics of La0.6Sr0.4CoO3−δ thin film electrodes. J. Electrochem. Soc. 158, B727–B734 (2011).
Téllez, H., Druce, J., Kilner, J. A. & Ishihara, T. Relating surface chemistry and oxygen surface exchange in LnBaCo2O5+δ air electrodes. Faraday Discuss. 182, 145–157 (2015).
Druce, J. et al. Surface termination and subsurface restructuring of perovskite-based solid oxide electrode materials. Energy Environ. Sci. 7, 3593–3599 (2014). This paper provides valuable insight into the surface reorganization in a range of perovskites relevant for SOC applications, which is essential for understanding and controlling surface-related phenomena at the electrochemical interface.
Zhang, C. et al. Measuring fundamental properties in operating solid oxide electrochemical cells by using in situ X-ray photoelectron spectroscopy. Nature Mater. 9, 944–949 (2010).
Li, X. et al. High-temperature surface enhanced Raman spectroscopy for in situ study of solid oxide fuel cell materials. Energy Environ. Sci. 7, 306–310 (2013). This paper reports on the development of high-temperature surface-enhanced Raman spectroscopy, which represents an important step towards in situ and in operando study of SOC electrode surfaces for obtaining mechanistic insight into electrochemical processes.
Choi, Y., Lin, M. C. & Liu, M. Rational design of novel cathode materials in solid oxide fuel cells using first-principles simulations. J. Power Sources 195, 1441–1445 (2010).
Liu, M., Lynch, M. E., Blinn, K., Alamgir, F. M. & Choi, Y. Rational SOFC material design: new advances and tools. Mater. Today 14, 534–546 (2011).
Traulsen, M. L. et al. Need for in operando characterization of electrochemical interface features. ECS Trans. 66, 3–20 (2015).
Hansen, K. V., Norrman, K., Jacobsen, T., Wu, Y. & Mogensen, M. B. LSM microelectrodes: kinetics and surface composition. J. Electrochem. Soc. 162, F1165–F1174 (2015).
Feng, Z. A., El Gabaly, F., Ye, X., Shen, Z.-X. & Chueh, W. C. Fast vacancy-mediated oxygen ion incorporation across the ceria–gas electrochemical interface. Nature Commun. 5, 4374 (2014).
Nowotny, J. Science of Ceramic Interfaces II (Newnes, 1995).
Lee, W., Han, J. W., Chen, Y., Cai, Z. & Yildiz, B. Cation size mismatch and charge interactions drive dopant segregation at the surfaces of manganite perovskites. J. Am. Chem. Soc. 135, 7909–7925 (2013). This paper provides a mechanistic model for the segregation and reorganization observed at the surface of perovskite materials, highlighting means to control them with anticipated benefits in enhancing the electrochemical activity of perovskite-based SOC electrodes.
Chen, Y. et al. Impact of Sr segregation on the electronic structure and oxygen reduction activity of SrTi1−xFexO3 surfaces. Energy Environ. Sci. 5, 7979–7988 (2012).
Szot, K. & Speier, W. Surfaces of reduced and oxidized SrTiO3 from atomic force microscopy. Phys. Rev. B 60, 5909–5926 (1999).
Baumann, F. S. et al. Strong performance improvement of La0.6Sr0.4Co0.8Fe0.2O3−δ SOFC cathodes by electrochemical activation. J. Electrochem. Soc. 152, A2074 (2005).
Tomkiewicz, A. C., Tamimi, M. A., Huq, A. & McIntosh, S. FD electrolysis: is the surface oxygen exchange rate linked to bulk ion diffusivity in mixed conducting Ruddlesden–Popper phases? Faraday Discuss. 182, 113–127 (2015).
Neagu, D. et al. Nano-socketed nickel particles with enhanced coking resistance grown in situ by redox exsolution. Nature Commun. 6, 8120 (2015). This paper compares the key structural and physicochemical properties of nickel metal particles prepared by deposition and exsolution methods, highlighting the latter as highly promising for enhancing the functionality of perovskite-supported metal particles for a range of applications including SOCs.
Chen, Y. et al. Advances in cathode materials for solid oxide fuel cells: complex oxides without alkaline earth metal elements. Adv. Energy Mater. http://doi.org/f28dmr (2015).
Jiang, S. P. & Chen, X. Chromium deposition and poisoning of cathodes of solid oxide fuel cells – a review. Int. J. Hydrog. Energy 39, 505–531 (2014).
Nowotny, J., Sorrell, C. C. & Bak, T. Segregation in zirconia: equilibrium versus non-equilibrium segregation. Surf. Interface Anal. 37, 316–324 (2005).
Hansen, K. V. & Mogensen, M. Absence of dopant segregation to the surface of scandia and yttria co-stabilized zirconia. Electrochem. Solid-State Lett. 15, B70–B71 (2012).
Mogensen, M. & Hansen, K. V. in Handbook of Fuel Cells: Advances in Electrocatalysis, Materials, Diagnostics and Durability (Wiley, 2009); http://go.nature.com/6RzEqt
Mogensen, M. & Holtappels, P. in Solid Oxide Fuels Cells: Facts and Figures (eds Irvine, J. T. S. & Connor, P. ) 25–45 (Springer, 2013).
Zhao, L., Perry, N. H., Daio, T., Sasaki, K. & Bishop, S. R. Improving the Si impurity tolerance of Pr0.1Ce0.9O2−δ SOFC electrodes with reactive surface additives. Chem. Mater. 27, 3065–3070 (2015).
Larsen, P. H., Mogensen, M., Hendriksen, P. V., Linderoth, S. & Ming, C. Removal of impurity phases from electrochemical devices. European patent 2031677 A1 (2009).
Backhaus-Ricoult, M. et al. In situ study of operating SOFC LSM/YSZ cathodes under polarization by photoelectron microscopy. Solid State Ion. 179, 891–895 (2008).
Knibbe, R., Traulsen, M. L., Hauch, A., Ebbesen, S. D. & Mogensen, M. Solid oxide electrolysis cells: degradation at high current densities. J. Electrochem. Soc. 157, B1209 (2010).
Chen, K. & Jiang, S. P. Failure mechanism of (La, Sr)MnO3 oxygen electrodes of solid oxide electrolysis cells. Int. J. Hydrog. Energy 36, 10541–10549 (2011).
Tietz, F., Sebold, D., Brisse, A. & Schefold, J. Degradation phenomena in a solid oxide electrolysis cell after 9000 h of operation. J. Power Sources 223, 129–135 (2013).
Graves, C., Ebbesen, S. D., Jensen, S. H., Simonsen, S. B. & Mogensen, M. B. Eliminating degradation in solid oxide electrochemical cells by reversible operation. Nature Mater. 14, 239–244 (2015). This paper demonstrates that electrolysis-induced degradation, previously believed to be irreversible, can in fact be reverted by cycling between electrolysis and fuel-cell modes, thus highlighting not only unexpected SOCs rejuvenation mechanisms for enhanced long-term stability, but also the viability of applying SOCs for renewable electricity storage.
Hughes, G. A., Railsback, J. G., Yakal-Kremski, K. J., Butts, D. M. & Barnett, S. A. Degradation of (La0.8Sr0.2)0.98MnO3−δ–Zr0.84Y0.16O2−γ composite electrodes during reversing current operation. Faraday Discuss. 182, 365–377 (2015).
la O', G. J., Savinell, R. F. & Shao-Horn, Y. Activity enhancement of dense strontium-doped lanthanum manganite thin films under cathodic polarization: a combined AES and XPS study. J. Electrochem. Soc. 156, B771 (2009).
Niakolas, D. K. Sulfur poisoning of Ni-based anodes for solid oxide fuel cells in H/C-based fuels. Appl. Catal. Gen. 486, 123–142 (2014).
Ebbesen, S. D., Graves, C., Hauch, A., Jensen, S. H. & Mogensen, M. Poisoning of solid oxide electrolysis cells by impurities. J. Electrochem. Soc. 157, B1419 (2010).
Pihlatie, M., Ramos, T. & Kaiser, A. Testing and improving the redox stability of Ni-based solid oxide fuel cells. J. Power Sources 193, 322–330 (2009).
Faes, A. et al. Design of experiment approach applied to reducing and oxidizing tolerance of anode supported solid oxide fuel cell. Part II: electrical, electrochemical and microstructural characterization of tape-cast cells. J. Power Sources 196, 8909–8917 (2011).
Graves, C. Reversing and repairing microstructure degradation in solid oxide cells during operation. ECS Trans. 57, 3127–3136 (2013).
Klotz, D. et al. Performance Enhancement of SOFC Anode Through Electrochemically Induced Ni/YSZ Nanostructures. J. Electrochem. Soc. 158, B587 (2011).
Graves, C., Chatzichristodoulou, C. & Mogensen, M. B. Kinetics of CO/CO2 and H2/H2O reactions at Ni-based and ceria-based solid-oxide-cell electrodes. Faraday Discuss. 182, 75–95 (2015).
Chen, M. et al. Microstructural degradation of Ni/YSZ electrodes in solid oxide electrolysis cells under high current. J. Electrochem. Soc. 160, F883–F891 (2013).
Baumann, F. S. et al. Quantitative comparison of mixed conducting SOFC cathode materials by means of thin film model electrodes. J. Electrochem. Soc. 154, B931–B941 (2007).
Abernathy, H. et al. Examination of the mechanism for the reversible aging behaviour at open circuit when changing the operating temperature of (La0.8Sr0.2)0.95MnO3 electrodes. Solid State Ion. 272, 144–154 (2015).
DeCaluwe, S. C. et al. In situ characterization of ceria oxidation states in high-temperature electrochemical cells with ambient pressure XPS. J. Phys. Chem. C 114, 19853–19861 (2010).
Molero-Sánchez, B., Addo, P., Buyukaksoy, A., Paulson, S. & Birss, V. Electrochemistry of La0.3Sr0.7Fe0.7 Cr0.3O3−δ as an oxygen and fuel electrode for RSOFCs. Faraday Discuss. 182, 159–175 (2015).
Blennow, P., Kammer Hansen, K., Wallenberg, L. R. & Mogensen, M. Strontium titanate-based composite anodes for solid oxide fuel cells. ECS Trans. 13, 181–194 (2008).
Corre, G. et al. Activation and ripening of impregnated manganese containing perovskite SOFC electrodes under redox cycling. Chem. Mater. 21, 1077–1084 (2009).
Lee, S.-Y. & Aris, R. The distribution of active ingredients in supported catalysts prepared by impregnation. Catal. Rev. 27, 207–340 (1985).
Komiyama, M. Design and preparation of impregnated catalysts. Catal. Rev. 27, 341–372 (1985).
Vohs, J. M. & Gorte, R. J. High-performance SOFC cathodes prepared by infiltration. Adv. Mater. 21, 943–956 (2009). This paper reviews the fabrication and modification of SOC electrodes by infiltration of active components into a porous scaffold, while providing insights into the relationships between the materials properties, electrochemical performance and stability.
Ding, D., Li, X., Lai, S. Y., Gerdes, K. & Liu, M. Enhancing SOFC cathode performance by surface modification through infiltration. Energy Environ. Sci. 7, 552–575 (2014).
Liu, Z. et al. Fabrication and modification of solid oxide fuel cell anodes via wet impregnation/infiltration technique. J. Power Sources 237, 243–259 (2013).
Jiang, S. P. Nanoscale and nano-structured electrodes of solid oxide fuel cells by infiltration: advances and challenges. Int. J. Hydrog. Energy 37, 449–470 (2012).
Zhao, L., Amarasinghe, S. & Jiang, S. P. Enhanced chromium tolerance of La0.6Sr0.4Co0.2Fe0.8O3−δ electrode of solid oxide fuel cells by Gd0.1Ce0.9O1.95 impregnation. Electrochem. Commun. 37, 84–87 (2013).
Lou, X. et al. Controlling the morphology and uniformity of a catalyst-infiltrated cathode for solid oxide fuel cells by tuning wetting property. J. Power Sources 195, 419–424 (2010).
Choi, Y. et al. Highly efficient layer-by-layer-assisted infiltration for high-performance and cost-effective fabrication of nanoelectrodes. ACS Appl. Mater. Interfaces 6, 17352–17357 (2014).
Sholklapper, T. Z., Jacobson, C. P., Visco, S. J. & De Jonghe, L. C. Synthesis of dispersed and contiguous nanoparticles in solid oxide fuel cell electrodes. Fuel Cells 8, 303–312 (2008).
Ni, C. S., Vohs, J. M., Gorte, R. J. & Irvine, J. T. S. Fabrication and characterisation of a large-area solid oxide fuel cell based on dual tape cast YSZ electrode skeleton supported YSZ electrolytes with vanadate and ferrite perovskite-impregnated anodes and cathodes. J. Mater. Chem. A 2, 19150–19155 (2014).
Savaniu, C.-D., Miller, D. N. & Irvine, J. T. S. Scale up and anode development for La-doped SrTiO3 anode-supported SOFCs. J. Am. Ceram. Soc. 96, 1718–1723 (2013).
Ramos, T. et al. Effect of Ru/CGO versus Ni/CGO co-infiltration on the performance and stability of STN-based SOFCs. Fuel Cells 14, 1062–1065 (2014).
Verbraeken, M. C. et al. Short stack and full system test using a ceramic A-site deficient strontium titanate anode. Fuel Cells 15, 682–688 (2015). This paper reports on the concept, fabrication and testing of the first all-oxide SOFC system at kW scale demonstrating the viability of this technology at an industrially relevant scale.
Shiozaki, R. et al. Partial oxidation of methane over a Ni/BaTiO3 catalyst prepared by solid phasecrystallization. J. Chem. Soc. Faraday Trans. 93, 3235–3242 (1997).
Nishihata, Y. et al. Self-regeneration of a Pd-perovskite catalyst for automotive emissions control. Nature 418, 164–167 (2002).
Madsen, B. D., Kobsiriphat, W., Wang, Y., Marks, L. D. & Barnett, S. SOFC anode performance enhancement through precipitation of nanoscale catalysts. ECS Trans. 7, 1339–1348 (2007).
Neagu, D., Tsekouras, G., Miller, D. N., Mé nard, H. & Irvine, J. T. S. In situ growth of nanoparticles through control of non-stoichiometry. Nature Chem. 5, 916–923 (2013).
Neagu, D. & Irvine, J. T. S. in Comprehensive Inorganic Chemistry II 2nd edn (eds Reedijk, J. & Poeppelmeier, K. ) 397–415 (Elsevier, 2013).
Tanaka, H. et al. The intelligent catalyst having the self-regenerative function of Pd, Rh and Pt for automotive emissions control. Catal. Today 117, 321–328 (2006).
Bierschenk, D. M. et al. Pd-substituted (La, Sr)CrO3−δ–Ce0.9Gd0.1O2−δ solid oxide fuel cell anodes exhibiting regenerative behavior. J. Power Sources 196, 3089–3094 (2011).
Tanaka, H. et al. Self-regenerating Rh- and Pt-based perovskite catalysts for automotive-emissions control. Angew. Chem. Int. Ed. 45, 5998–6002 (2006).
Kobsiriphat, W. et al. Nickel- and ruthenium-doped lanthanum chromite anodes: effects of nanoscale metal precipitation on solid oxide fuel cell performance. J. Electrochem. Soc. 157, B279–B284 (2010).
Jardiel, T. et al. New SOFC electrode materials: the Ni-substituted LSCM-based compounds (La0.75Sr0.25)(Cr0.5Mn0.5−xNix)O3−δ and (La0.75Sr0.25)(Cr0.5−xNixMn0.5)O3−δ . Solid State Ion. 181, 894–901 (2010).
Sun, Y. et al. A-site deficient perovskite: the parent for in situ exsolution of highly active, regenerable nano-particles as SOFC anodes. J. Mater. Chem. A 3, 11048–11056 (2015).
Tsekouras, G., Neagu, D. & Irvine, J. T. S. Step-change in high temperature steam electrolysis performance of perovskite oxide cathodes with exsolution of B-site dopants. Energy Environ. Sci. 6, 256–266 (2013). This paper shows the use of in situ exsolution of metal particles from tailored perovskite electrodes to enhance high-temperature steam electrolysis in a SOEC, thus providing proof of concept for more efficient preparation and operation of SOEC electrodes for energy storage.
Boulfrad, S., Cassidy, M., Djurado, E., Irvine, J. T. S. & Jabbour, G. Pre-coating of LSCM perovskite with metal catalyst for scalable high performance anodes. Int. J. Hydrog. Energy 38, 9519–9524 (2013).
Yang, C. et al. In situ fabrication of CoFe alloy nanoparticles structured (Pr0.4Sr0.6)3(Fe0.85Nb0.15)2O7 ceramic anode for direct hydrocarbon solid oxide fuel cells. Nano Energy 11, 704–710 (2015).
Qin, Q. et al. Perovskite titanate cathode decorated by in situ grown iron nanocatalyst with enhanced electrocatalytic activity for high-temperature steam electrolysis. Electrochimica Acta 127, 215–227 (2014).
Abild-Pedersen, F., Nørskov, J. K., Rostrup-Nielsen, J. R., Sehested, J. & Helveg, S. Mechanisms for catalytic carbon nanofiber growth studied by ab initio density functional theory calculations. Phys. Rev. B 73, 115419 (2006).
Katz, M. B. et al. Reversible precipitation/dissolution of precious-metal clusters in perovskite-based catalyst materials: bulk versus surface re-dispersion. J. Catal. 293, 145–148 (2012).
Vels Jensen, K., Primdahl, S., Chorkendorff, I. & Mogensen, M. Microstructural and chemical changes at the Ni/YSZ interface. Solid State Ion. 144, 197–209 (2001).
Hansen, K. V., Norrman, K. & Mogensen, M. H2 H2O Ni YSZ electrode performance effect of segregation to the interface. J. Electrochem. Soc. 151, A1436–A1444 (2004).
Verbraeken, M. C., Iwanschitz, B., Mai, A. & Irvine, J. T. S. Evaluation of Ca doped La0.2Sr0.7TiO3 as an alternative material for use in SOFC anodes. J. Electrochem. Soc. 159, F757–F762 (2012).
Baumann, F. S., Fleig, J., Habermeier, H.-U. & Maier, J. Impedance spectroscopic study on well-defined (La, Sr)(Co, Fe)O3−δ model electrodes. Solid State Ion. 177, 1071–1081 (2006).
Kuklja, M. M., Kotomin, E. A., Merkle, R., Mastrikov, Y. A. & Maier, J. Combined theoretical and experimental analysis of processes determining cathode performance in solid oxide fuel cells. Phys. Chem. Chem. Phys. 15, 5443–5471 (2013).
Mueller, D. N., Machala, M. L., Bluhm, H. & Chueh, W. C. Redox activity of surface oxygen anions in oxygen-deficient perovskite oxides during electrochemical reactions. Nature Commun. 6, 6097 (2015).
Sase, M. et al. Enhancement of oxygen exchange at the hetero interface of (La, Sr)CoO3/(La, Sr)2CoO4 in composite ceramics. Solid State Ion. 178, 1843–1852 (2008).
Mogensen, M., Høgh, J., Hansen, K. V. & Jacobsen, T. A critical review of models of the H2/H2O/Ni/SZ electrode kinetics. ECS Trans. 7, 1329–1338 (2007).
Chueh, W. C., Hao, Y., Jung, W. & Haile, S. M. High electrochemical activity of the oxide phase in model ceria–Pt and ceria–Ni composite anodes. Nature Mater. 11, 155–161 (2012).
Chen J. et al. Performance of large-scale anode-supported solid oxide fuel cells with impregnated La0.6Sr0.4Co0.2Fe0.8O3−δ+Y2O3 stabilized ZrO2 composite cathodes. J. Power Sources 195, 5201–5205 (2010).
Acknowledgements
C.C. acknowledges financial support from ECoProbe (DFF – 4005-00129) funded by the Danish Independent Research Council. C.G. and M.B.M. acknowledge financial support from Energinet.dk through the ForskEL programme Solid Oxide Fuel Cells for the Renewable Energy Transition contract no. 2014-1-12231. J.T.S.I., M.C.V. and D.N. acknowledge support from EPSRC Platform Grant EP/K015540/1, EPSRC Tailoring of microstructural evolution in impregnated SOFC electrodes EP/M014304/1 and Royal Society Wolfson Merit Award WRMA 2012/R2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Irvine, J., Neagu, D., Verbraeken, M. et al. Evolution of the electrochemical interface in high-temperature fuel cells and electrolysers. Nat Energy 1, 15014 (2016). https://doi.org/10.1038/nenergy.2015.14
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nenergy.2015.14
This article is cited by
-
Accelerated stress test protocol for solid oxide cell based on ex-situ artificial aging of the fuel electrode via redox-cycling - In memory of Prof. A. Milchev
Journal of Solid State Electrochemistry (2024)
-
NaNO3-mediated synthesis of Pt-doped titanate perovskites for oxygen-reduction reactions in acidic media
Journal of Sol-Gel Science and Technology (2023)
-
The review of advances in interfacial electrochemistry in Estonia: electrochemical double layer and adsorption studies for the development of electrochemical devices
Journal of Solid State Electrochemistry (2023)
-
Reversely trapping atoms from a perovskite surface for high-performance and durable fuel cell cathodes
Nature Catalysis (2022)
-
Anti-phase boundary accelerated exsolution of nanoparticles in non-stoichiometric perovskite thin films
Nature Communications (2022)