Abstract
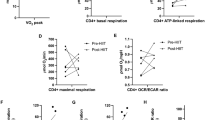
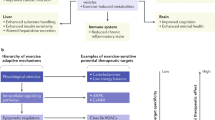
Exercise is now known to be beneficial for patients with inflammatory rheumatic disease. In patients with rheumatoid arthritis, exercise can improve physical performance, cardiorespiratory fitness and muscle strength, and reduce disease activity and systemic inflammation, as evidenced by reductions in erythrocyte sedimentation rate and other systemic markers of inflammation. Similar effects on physical performance and cardiorespiratory fitness have been observed in patients with polymyositis and dermatomyositis. Improved muscle performance in these patients is associated with an increased ratio of type I : type II muscle fibers and increased cross-sectional area of type II muscle fibers, suggesting that myositis-affected muscle retains the ability to respond to exercise. In addition, resistance exercise training can reduce the expression of genes involved in inflammation and fibrosis in patients with myositis, and in vitro mechanical loading of chondrocytes can suppress the expression of proinflammatory cytokines, indicating that exercise can also reduce inflammation in the local tissue environment. Further studies of the systemic and local responses underlying exercise-associated improvement in muscle performance, soft tissue integrity and health outcomes are warranted.
Key Points
-
Impaired muscle performance and muscle atrophy are common features in patients with chronic rheumatic disorders
-
Exercise training can improve performance without exacerbation of disease progress
-
Exercise training can reduce systemic inflammation
-
Exercise training might have beneficial effects on certain molecular processes in muscle tissue and cartilage that reduce inflammation and fibrosis and promote tissue repair
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ekblom B et al. (1975) Effect of short-term physical training on patients with rheumatoid arthritis I. Scand J Rheumatol 4: 80–86
Stenström CH and Minor MA (2003) Evidence for the benefit of aerobic and strengthening exercise in rheumatoid arthritis. Arthritis Rheum 49: 428–434
Roos EM and Dahlberg L (2005) Positive effects of moderate exercise on glycosaminoglycan content in knee cartilage: a four-month, randomized, controlled trial in patients at risk of osteoarthritis. Arthritis Rheum 52: 3507–3514
Hicks JE et al. (1993) Isometric exercise increases strength and does not produce sustained creatinine phosphokinase increases in a patient with polymyositis. J Rheumatol 20: 1399–1401
Escalante A et al. (1993) Resistive exercise in the rehabilitation of polymyositis/dermatomyositis. J Rheumatol 20: 1340–1344
Wiesinger GF et al. (1998) Improvement of physical fitness and muscle strength in polymyositis/dermatomyositis patients by a training programme. Br J Rheumatol 37: 196–200
Wiesinger GF et al. (1998) Benefit of 6 months long-term physical training in polymyositis/dermatomyositis patients. Br J Rheumatol 37: 1338–1342
Alexanderson H et al. (1999) Safety of a home exercise programme in patients with polymyositis and dermatomyositis: a pilot study. Rheumatology (Oxford) 38: 608–611
Alexanderson H et al. (2000) The safety of a resistive home exercise program in patients with recent onset active polymyositis or dermatomyositis. Scand J Rheumatol 29: 295–301
Varjú C et al. (2003) The effect of physical exercise following acute disease exacerbation in patients with dermato/polymyositis. Clin Rehabil 17: 83–87
Harris-Love MO (2005) Safety and efficacy of submaximal eccentric strength training for a subject with polymyositis. Arthritis Rheum 53: 471–474
Alexanderson H et al. (2007) Benefits of intensive resistance training in patients with chronic polymyositis or dermatomyositis. Arthritis Rheum 57: 768–777
Häkkinen A et al. (2003) Effects of concurrent strength and endurance training in women with early or longstanding rheumatoid arthritis: comparison with healthy subjects. Arthritis Rheum 49: 789–797
Häkkinen A et al. (2001) A randomized two-year study of the effects of dynamic strength training on muscle strength, disease activity, functional capacity, and bone mineral density in early rheumatoid arthritis. Arthritis Rheum 44: 515–522
van den Ende CH et al. (2000) Effect of intensive exercise on patients with active rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 59: 615–621
Rall LC et al. (1996) Protein metabolism in rheumatoid arthritis and aging. Effects of muscle strength training and tumor necrosis factor alpha. Arthritis Rheum 39: 1115–1124
Fischer CP et al. (2007) Plasma levels of interleukin-6 and C-reactive protein are associated with physical inactivity independent of obesity. Scand J Med Sci Sports 17: 580–587
Olson TP et al. (2007) Changes in inflammatory biomarkers following one-year of moderate resistance training in overweight women. Int J Obes (Lond) 31: 996–1003
Dekker MJ et al. (2007) An exercise intervention without weight loss decreases circulating interleukin-6 in lean and obese men with and without type 2 diabetes mellitus. Metabolism 56: 332–338
Kullo IJ et al. (2007) Markers of inflammation are inversely associated with VO2 max in asymptomatic men. J Appl Physiol 102: 1374–1379
Wolfe F et al. (2003) Increase in cardiovascular and cerebrovascular disease prevalence in rheumatoid arthritis. J Rheumatol 30: 36–40
Wållberg-Jonsson S et al. (1999) Extent of inflammation predicts cardiovascular disease and overall mortality in seropositive rheumatoid arthritis. A retrospective cohort study from disease onset. J Rheumatol 26: 2562–2571
Sokka T et al. (2008) Physical inactivity in patients with rheumatoid arthritis: data from twenty-one countries in a cross-sectional, international study. Arthritis Rheum 59: 42–50
Halla JT et al. (1984) Rheumatoid myositis. Clinical and histologic features and possible pathogenesis. Arthritis Rheum 27: 737–743
Nordemar R et al. (1976) Changes in muscle fibre size and physical performance in patients with rheumatoid arthritis after 7 months physical training. Scand J Rheumatol 5: 233–238
Reid MB et al. (2002) Respiratory and limb muscle weakness induced by tumor necrosis factor-alpha: involvement of muscle myofilaments. Am J Respir Crit Care Med 166: 479–484
Nagaraju K et al. (2005) Activation of the endoplasmic reticulum stress response in autoimmune myositis: potential role in muscle fiber damage and dysfunction. Arthritis Rheum 52: 1824–1835
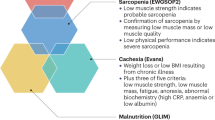
Walsmith J and Roubenoff R (2002) Cachexia in rheumatoid arthritis. Int J Cardiol 85: 89–99
Baracos V et al. (1983) Stimulation of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). A mechanism for the increased degradation of muscle proteins during fever. N Engl J Med 308: 553–558
Moldawer LL and Copeland EM III (1997) Proinflammatory cytokines, nutritional support, and the cachexia syndrome: interactions and therapeutic options. Cancer 79: 1828–1839
Nader GA (2005) Molecular determinants of skeletal muscle mass: getting the “AKT” together. Int J Biochem Cell Biol 37: 1985–1996
Voisin L et al. (1996) Muscle wasting in a rat model of long-lasting sepsis results from the activation of lysosomal, Ca2-activated, and ubiquitin-proteasome proteolytic pathways. J Clin Invest 97: 1610–1617
Huang J and Forsberg NE (1999) Role of calpain in skeletal-muscle protein degradation. Proc Natl Acad Sci USA 95: 12100–12105
Du J et al. (2004) Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest 113: 115–123
Lecker SH et al. (1999) Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr 12: 227–237
Guttridge DC (2004) Signaling pathways weigh in on decisions to make or break skeletal muscle. Curr Opin Clin Nutr Metab Care 7: 443–450
Ladner KJ et al. (2003) Tumor necrosis factor-regulated biphasic activation of NF-kappa B is required for cytokine-induced loss of skeletal muscle gene products. J Biol Chem 278: 2294–2303
Marcora SM et al. (2006) Randomized phase 2 trial of anti-tumor necrosis factor therapy for cachexia in patients with early rheumatoid arthriti. Am J Clin Nutr 84: 1463–1472
Granado M et al. (2006) Tumor necrosis factor blockade did not prevent the increase of muscular muscle RING finger-1 and muscle atrophy F-box in arthritic rats. J Endocrinol 191: 319–326
Efthimiou P et al. (2006) Possible role for tumour necrosis factor inhibitors in the treatment of resistant dermatomyositis and polymyositis: a retrospective study of eight patients. Ann Rheum Dis 65: 1233–1236
Dastmalchi M et al. (2008) A high incidence of disease flares in an open pilot study of infliximab in patients with refractory inflammatory myopathies. Ann Rheum Dis [10.1136/ard.2007.077974]
McDonagh MJ and Davies CT (1984) Adaptive response of mammalian skeletal muscle to exercise with high loads. Eur J Appl Physiol Occup Physiol 52: 139–155
Bergström J (1962) Muscle electrolytes in man. Scand J Clin Lab Med 14: 511–513
Malm C et al. (2000) Immunological changes in human skeletal muscle and blood after eccentric exercise and multiple biopsies. J Physiol 529: 243–262
Henriksson KG (1979) “Semi-open” muscle biopsy technique. A simple outpatient procedure. Acta Neurol Scand 59: 317–323
Nyberg P et al. (2000) Increased expression of interleukin 1α and MHC class I in muscle tissue of patients with chronic, inactive polymyositis and dermatomyositis. J Rheumatol 27: 940–948
Dastmalchi M et al. (2007) Effect of physical training on the proportion of slow-twitch type I muscle fibers, a novel nonimmune-mediated mechanism for muscle impairment in polymyositis or dermatomyositis. Arthritis Rheum 57: 1303–1310
Park JH et al. (1994) Magnetic resonance imaging and p-31 magnetic resonance spectroscopy provide unique quantitative data useful in the longitudinal management of patients with dermatomyositis. Arthritis Rheum 37: 736–746
Lundberg I et al. (1997) Cytokine production in muscle tissue in idiopathic inflammatory myopathies. Arthritis Rheum 40: 865–874
Ulfgren AK et al. (2004) Down-regulation of the aberrant expression of the inflammation mediator high mobility group box chromosomal protein 1 in muscle tissue of patients with polymyositis and dermatomyositis treated with corticosteroids. Arthritis Rheum 50: 1586–1594
Radom-Aizik S et al. (2007) Effects of exercise training on quadriceps muscle gene expression in chronic obstructive pulmonary disease. J Appl Physiol 102: 1976–1984
Gielen S et al. (2003) Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol 42: 861–868
Chung YL et al. (2007) Creatine supplements in patients with idiopathic inflammatory myopathies who are clinically weak after conventional pharmacologic treatment: six-month, double-blind, randomized, placebo-controlled trial. Arthritis Rheum 57: 694–702
Gassner R et al. (1999) Cyclic tensile stress exerts anti-inflammatory actions on chondrocytes by inhibiting inducible nitric oxide synthase. J Immunol 163: 2187–2192
Xu Z et al. (2000) Cyclic tensile strain acts as an antagonist of IL-1 beta actions in chondrocytes. J Immunol 165: 453–460
Ferretti M et al. (2006) Dynamic biophysical strain modulates proinflammatory gene induction in meniscal fibrochondrocytes. Am J Physiol Cell Physiol 290: C1610–C1615
Madhavan S et al. (2006) Biomechanical signals exert sustained attenuation of proinflammatory gene induction in articular chondrocytes. Osteoarthritis Cartilage 14: 1023–1032
Dossumbekova A et al. (2007). Biomechanical signals inhibit IKK activity to attenuate NF-kappaB transcription activity in inflamed chondrocytes. Arthritis Rheum 56: 3284–3296
Acknowledgements
The authors apologize to those colleagues whose important work could not be cited due to space restrictions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lundberg, I., Nader, G. Molecular effects of exercise in patients with inflammatory rheumatic disease. Nat Rev Rheumatol 4, 597–604 (2008). https://doi.org/10.1038/ncprheum0929
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncprheum0929
This article is cited by
-
Effectiveness of aquatic exercise in the treatment of inflammatory arthritis: systematic review
Rheumatology International (2022)
-
Feasibility of a blended therapy approach in the treatment of patients with inflammatory myopathies
Archives of Physiotherapy (2021)
-
Evaluation of a special concept of physical therapy in spondyloarthritis: German multimodal rheumatologic complex treatment for spondyloarthritis
Clinical Rheumatology (2020)
-
Strategies for optimising musculoskeletal health in the 21st century
BMC Musculoskeletal Disorders (2019)
-
The immune system in sporadic inclusion body myositis patients is not compromised by blood-flow restricted exercise training
Arthritis Research & Therapy (2019)