Abstract
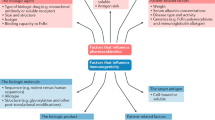
Antibody engineering and protein design have led to the creation of a new era of targeted anti-inflammatory therapies in rheumatology. Recombinant DNA technologies have enabled the selection and humanization of specific antibody fragments in order to develop therapeutic reagents of any specificity that can be 'armed' to deliver effective anti-inflammatory 'payloads'. Antibodies and antibody-like proteins provide the opportunity to block key soluble mediators of inflammation in their milieu, or alternatively to block intracellular inflammation-triggering pathways by binding to an upstream cell-surface receptor. These designer proteins can be tuned for desired pharmacokinetic and pharmacodynamic effects, and represent tools for specific therapeutic intervention by delivering precisely the required immunosuppressive effect. The extent of desired and undesired effects of a particular biologic therapy, however, can be broader than initially predicted and require careful evaluation during clinical trials. This Review highlights advances in recombinant technologies for the development of novel biologic therapies in rheumatology.
Key Points
-
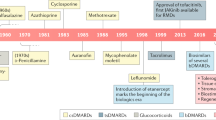
Led by the successful wave of anti-inflammatory modulators in rheumatic disease therapy, designed protein therapeutic agents now outnumber and surpass the number of small-molecule drugs approved annually by the FDA
-
Antibodies and immunoadhesins that directly target cytokines for their systemic removal (ligand ablation; e.g. adalinmumab, infliximab) have become effective therapeutic strategies in rheumatology
-
Novel and powerful protein display technologies have enabled the rapid isolation of fully human therapeutics, often with unique disease-targeting specificities
-
Engineered antibodies have become essential therapies against a range of rheumatic diseases, and future product designs will offer enhanced clinical efficacy with fewer adverse events
-
By incorporating our in-depth knowledge of protein structure, the future impact of novel engineered biologic agents as treatments for rheumatic diseases will be immense
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Olafsen T et al. (2006) Tunable pharmacokinetics: modifying the in vivo half-life of antibodies by directed mutagenesis of the Fc fragment. Nat Protoc 1: 2048–2060
Walsh G and Jefferis R (2006) Post-translational modifications in the context of therapeutic proteins. Nat Biotechnol 24: 1241–1252
Holliger P and Hudson PJ (2005) Engineered antibody fragments and the rise of single domains. Nat Biotechnol 23: 1126–1136
Lipovsek D et al. (2007) Evolution of an interloop disulfide bond in high-affinity antibody mimics based on fibronectin type III domain and selected by yeast surface display: molecular convergence with single-domain camelid and shark antibodies. J Mol Biol 368: 1024–1041
Cohen SB et al. (2004) A multicentre, double blind, randomised, placebo controlled trial of anakinra (Kineret), a recombinant interleukin 1 receptor antagonist, in patients with rheumatoid arthritis treated with background methotrexate. Ann Rheum Dis 63: 1062–1068
Hosse RJ et al. (2006) A new generation of protein display scaffolds for molecular recognition. Protein Sci 15: 14–27
Moreland LW et al. (1997) Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)–Fc fusion protein. N Engl J Med 337: 141–147
Wang H et al. (2001) TACI-ligand interactions are required for T cell activation and collagen-induced arthritis in mice. Nat Immunol 2: 632–637
Mosquera LA et al. (2005) In vitro and in vivo characterization of a novel antibody-like single-chain TCR human IgG1 fusion protein. J Immunol 174: 4381–4388
Lonberg N (2005) Human antibodies from transgenic animals. Nat Biotechnol 23: 1117–1125
Hoogenboom HR (2005) Selecting and screening recombinant antibody libraries. Nat Biotechnol 23: 1105–1116
Rothe A et al. (2006) In vitro display technologies reveal novel biopharmaceutics. FASEB J 20: 1599–1610
Lipovsek D and Pluckthun A (2004) In-vitro protein evolution by ribosome display and mRNA display. J Immunol Methods 290: 51–67
Marks JD (2004) Antibody affinity maturation by chain shuffling. Methods Mol Biol 248: 327–343
Valjakka J et al. (2002) Crystal structure of an in vitro affinity- and specificity-matured anti-testosterone Fab in complex with testosterone. Improved affinity results from small structural changes within the variable domains. J Biol Chem 277: 44021–44027
Baker KP et al. (2003) Generation and characterization of LymphoStat-B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis Rheum 48: 3253–3265
Bayry J et al. (2007) Monoclonal antibody and intravenous immunoglobulin therapy for rheumatic diseases: rationale and mechanisms of action. Nat Clin Pract Rheumatol 3: 262–272
Chambers RS (2005) High-throughput antibody production. Curr Opin Chem Biol 9: 46–50
Kenanova V et al. (2007) Radioiodinated versus radiometal-labeled anti-carcinoembryonic antigen single-chain Fv-Fc antibody fragments: optimal pharmacokinetics for therapy. Cancer Res 67: 718–726
Ward ES et al. (2005) From sorting endosomes to exocytosis: association of Rab4 and Rab11 GTPases with the Fc receptor, FcRn, during recycling. Mol Biol Cell 16: 2028–2038
Woof JM and Burton DR (2004) Human antibody–Fc receptor interactions illuminated by crystal structures. Nat Rev Immunol 4: 89–99
Cartron G et al. (2007) Pharmacokinetics of rituximab and its clinical use: thought for the best use. Crit Rev Oncol Hematol 62: 43–52
Brocchini S et al. (2006) PEGylation of native disulfide bonds in proteins. Nat Protoc 1: 2241–2252
Choy EH et al. (2002) Efficacy of a novel PEGylated humanized anti-TNF fragment (CDP870) in patients with rheumatoid arthritis: a phase II double-blinded, randomized, dose-escalating trial. Rheumatology (Oxford) 41: 1133–1137
Rao BM et al. (2005) Integrating cell-level kinetic modeling into the design of engineered protein therapeutics. Nat Biotechnol 23: 191–194
Adams GP et al. (2001) High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules. Cancer Res 61: 4750–4755
Traggiai E et al. (2004) Development of a human adaptive immune system in cord blood cell-transplanted mice. Science 304: 104–107
De Groot AS and Moise L (2007) Prediction of immunogenicity for therapeutic proteins: state of the art. Curr Opin Drug Discov Devel 10: 332–340
Silverman GJ and Boyle DL (2008) Understanding the mechanistic basis in rheumatoid arthritis for clinical response to anti-CD20 therapy: the B-cell roadblock hypothesis. Immunol Rev 223: 175–185
Taylor RP and Lindorfer MA (2007) Drug Insight: the mechanism of action of rituximab in autoimmune disease—the immune complex decoy hypothesis. Nat Clin Pract Rheumatol 3: 86–95
Beers SA et al. (2008) Type II (tositumomab) anti-CD20 monoclonal antibody out performs Type I (rituximab-like) reagents in B-cell depletion regardless of complement activation. Blood [10.1182/blood-2008-04-149161]
Schett G et al. (2005) Mechanisms of Disease: the link between RANKL and arthritic bone disease. Nat Clin Pract Rheumatol 1: 47–54
Miller PD et al. (2008) Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone 43: 222–229
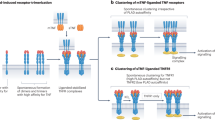
Tarner IH et al. (2007) Emerging targets of biologic therapies for rheumatoid arthritis. Nat Clin Pract Rheumatol 3: 336–345
Kunisch E et al. (2007) Predominant activation of MAP kinases and pro-destructive/pro-inflammatory features by TNF alpha in early-passage synovial fibroblasts via TNF receptor-1: failure of p38 inhibition to suppress matrix metalloproteinase-1 in rheumatoid arthritis. Ann Rheum Dis 66: 1043–1051
Strand V et al. (2007) Biologic therapies in rheumatology: lessons learned, future directions. Nat Rev Drug Discov 6: 75–92
van Vollenhoven R et al. (2003) Treatment with infliximab (Remicade) when etanercept (Enbrel) has failed or vice versa: data from the STURE registry showing that switching tumour necrosis factor alpha blockers can make sense. Ann Rheum Dis 62: 1195–1198
Cobo-Ibanez T and Martin-Mola E (2007) Etanercept: long-term clinical experience in rheumatoid arthritis and other arthritis. Expert Opin Pharmacother 8: 1373–1397
Sandborn WJ et al. (2007) Certolizumab pegol for the treatment of Crohn's disease. N Engl J Med 357: 228–238
Arana announces start of Phase II trial for lead compound. [http://www.arana.com/text/news_media/2008_html/ press_release_170308.html] (accessed 14 August 2008)
Holt LJ et al. (2008) Anti-serum albumin domain antibodies for extending the half-lives of short lived drugs. Protein Eng Des Sel 21: 283–288
Seyler TM et al. (2005) BLyS and APRIL in rheumatoid arthritis. J Clin Invest 115: 3083–3092
Ding C and Jones G (2006) Belimumab Human Genome Sciences/Cambridge Antibody Technology/GlaxoSmithKline. Curr Opin Investig Drugs 7: 464–472
Human genome sciences reports positive long-term data for LymphoStat-B in patients with active systemic lupus erythematosus. [http://www.hgsi.com/latest/human-genome-sciences-reports-positive-long- term-data-for-lymphostat-b-in-patients-with-active-systemic-lupus-erythema- 6.html] (accessed 14 August 2008)
Lin WY et al. (2007) Anti-BR3 antibodies: a new class of B-cell immunotherapy combining cellular depletion and survival blockade. Blood 110: 3959–3967
Nishimoto N et al. (2007) Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an X-ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis 66: 1162–1167
Ferrari-Lacraz S et al. (2004) Targeting IL-15 receptor-bearing cells with an antagonist mutant IL-15/Fc protein prevents disease development and progression in murine collagen-induced arthritis. J Immunol 173: 5818–5826
Baslund B et al. (2005) Targeting interleukin-15 in patients with rheumatoid arthritis: a proof-of-concept study. Arthritis Rheum 52: 2686–2692
Bruce SP and Boyce EG (2007) Update on abatacept: a selective costimulation modulator for rheumatoid arthritis. Ann Pharmacother 41: 1153–1162
Suntharalingam G et al. (2006) Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med 355: 1018–1028
Lainer-Carr D and Brahn E (2007) Angiogenesis inhibition as a therapeutic approach for inflammatory synovitis. Nat Clin Pract Rheumatol 3: 434–442
Strunk J et al. (2006) Anti-TNF-alpha antibody Infliximab and glucocorticoids reduce serum vascular endothelial growth factor levels in patients with rheumatoid arthritis: a pilot study. Rheumatol Int 26: 252–256
Holash J et al. (2002) VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA 99: 11393–11398
Hurwitz H et al. (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350: 2335–2342
Parker MH et al. (2005) Antibody mimics based on human fibronectin type three domain engineered for thermostability and high-affinity binding to vascular endothelial growth factor receptor two. Protein Eng Des Sel 18: 435–444
Acknowledgements
A Rothe is supported by Deutsche Krebshilfe and the Köln Fortune Foundation/Germany whilst working at the Commonwealth Scientific and Industrial Research Organisation (CSIRO). In addition, we acknowledge our scientific colleagues in our three primary institutions with whom we have enjoyed many exciting discussions on the new wave of therapeutic anti-inflammatory proteins.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
BE Power is an employee and stockholder in Patrys Ltd. PJ Hudson and BE Power are both founding Directors and stockholders in AntibOZ Pty Ltd and PJ Hudson is CEO and a stockholder in AviPep Pty Ltd.
Rights and permissions
About this article
Cite this article
Rothe, A., Power, B. & Hudson, P. Therapeutic advances in rheumatology with the use of recombinant proteins. Nat Rev Rheumatol 4, 605–614 (2008). https://doi.org/10.1038/ncprheum0909
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncprheum0909
This article is cited by
-
Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment
Nature Reviews Rheumatology (2017)
-
Biologika
Zeitschrift für Rheumatologie (2016)
-
Cell biology of osteoimmunology
Wiener Medizinische Wochenschrift (2010)