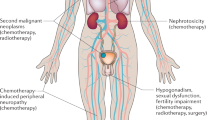
Abstract
Many cancer treatments induce gonadal failure, which can cause infertility and menopausal symptoms in women. Improvements in treatments for hematologic malignancies have extended survival, thus making treatment-induced gonadal failure (TIGF) a more widespread problem. We reviewed the published literature on TIGF with the goal of providing practical information for health-care providers engaged in the management of hematologic malignancies. We conclude that managing TIGF involves risk assessment, provision of information, discussion of potential options for preserving fertility, and referral to appropriate specialists. All patients with hematologic malignancies should be given information regarding TIGF at the earliest possible time, ideally before treatment begins.
Key Points
-
Managing treatment-induced gonadal failure involves risk assessment, provision of information, discussions of options for the preservation of fertility, and referral to appropriate specialists
-
Risk assessment should be done on an individual basis with consideration of the patient's age and sex, the type and status of disease, number of children, and other factors such as the wish to have children
-
The availability of fertility preservation should be discussed at the earliest possible opportunity, ideally before commencement of treatment
-
The use of high-dose alkylating agents and high-dose total-body irradiation as conditioning for hematopoietic stem-cell transplantation carry the highest risk of subsequent treatment-induced gonadal failure
-
Sperm banking and embryo cryopreservation are the most well-established options for preserving fertility in adult patients; options for prepubertal children have yet to be established
-
Teams consisting of physicians, nurses, pharmacists, psychologists, and reproductive specialists should work together to provide the necessary information to help patients make decisions regarding the options available to them
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Jemal A et al. (2007) Cancer statistics, 2007. CA Cancer J Clin 57: 43–66
Surveillance, Epidemiology, and End Results (SEER) program: SEER Cancer Statistics Review, 1975–2003 [http://seer.cancer.gov/csr/1975_2003/] (accessed October 27, 2006)
Schover LR (2005) Motivation for parenthood after cancer: a review. J Natl Cancer Inst Monogr 2–5
Schover LR et al. (1999) Having children after cancer. A pilot survey of survivors' attitudes and experiences. Cancer 86: 697–709
Lee SJ et al. (2006) American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol 24: 2917–2931
Duffy CM et al. (2005) Discussions regarding reproductive health for young women with breast cancer undergoing chemotherapy. J Clin Oncol 23: 766–773
Harris RP et al. (2001) Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med 20: 21–35
Sklar C (2005) Maintenance of ovarian function and risk of premature menopause related to cancer treatment. J Natl Cancer Inst Monogr 25–27
Sanders JE et al. (1988) Ovarian function following marrow transplantation for aplastic anemia or leukemia. J Clin Oncol 6: 813–818
Andrieu JM and Ochoa-Molina ME (1983) Menstrual cycle, pregnancies and offspring before and after MOPP therapy for Hodgkin's disease. Cancer 52: 435–438
Rivkees SA and Crawford JD (1988) The relationship of gonadal activity and chemotherapy-induced gonadal damage. JAMA 259: 2123–2125
Sklar CA et al. (2006) Premature menopause in survivors of childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst 98: 890–896
Lantinga GM et al. (2006) Imminent ovarian failure in childhood cancer survivors. Eur J Cancer 42: 1415–1420
van der Kaaij MA et al. (2007) Gonadal function in males after chemotherapy for early-stage Hodgkin's lymphoma treated in four subsequent trials by the European Organisation for Research and Treatment of Cancer: EORTC Lymphoma Group and the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol 25: 2825–2832
Ortin TT et al. (1990) Gonadal status and reproductive function following treatment for Hodgkin's disease in childhood: the Stanford experience. Int J Radiat Oncol Biol Phys 19: 873–880
Rueffer U et al. (2001) Male gonadal dysfunction in patients with Hodgkin's disease prior to treatment. Ann Oncol 12: 1307–1311
Fitoussi et al. (2000) Semen analysis and cryoconservation before treatment in Hodgkin's disease. Ann Oncol 11: 679–684
Blackhall FH et al. (2002) Semen cryopreservation, utilisation and reproductive outcome in men treated for Hodgkin's disease. Br J Cancer 87: 381–384
Hallak J et al. (1999) Cryopreservation of sperm from patients with leukemia: is it worth the effort? Cancer 85: 1973–1978
Bahadur G et al. (2002) Semen quality and cryopreservation in adolescent cancer patients. Hum Reprod 17: 3157–3161
Hallak J et al. (2000) Characteristics of cryopreserved semen from men with lymphoma. J Assist Reprod Genet 17: 591–594
Botchan A et al. (1997) Sperm quality in Hodgkin's disease versus non-Hodgkin's lymphoma. Hum Reprod 12: 73–76
Marmor D et al. (1986) Semen analysis in Hodgkin's disease before the onset of treatment. Cancer 57: 1986–1987
Redman JR et al. (1987) Semen cryopreservation and artificial insemination for Hodgkin's disease. J Clin Oncol 5: 233–238
Barr RD et al. (1993) Dyspermia in men with localized Hodgkin's disease. A potentially reversible, immune-mediated disorder. Med Hypotheses 40: 165–168
Kobayashi H et al. (2001) DNA damage in patients with untreated cancer as measured by the sperm chromatin structure assay. Fertil Steril 75: 469–475
O'Donovan M (2005) An evaluation of chromatin condensation and DNA integrity in the spermatozoa of men with cancer before and after therapy. Andrologia 37: 83–90
Sanders JE et al. (1983) Late effects on gonadal function of cyclophosphamide, total-body irradiation, and marrow transplantation. Transplantation 36: 252–255
Whitehead E et al. (1982) The effects of Hodgkin's disease and combination chemotherapy on gonadal function in the adult male. Cancer 49: 418–422
Viviani S et al. (1985) Gonadal toxicity after combination chemotherapy for Hodgkin's disease. Comparative results of MOPP vs ABVD. Eur J Cancer Clin Oncol 21: 601–605
Whitehead E et al. (1983) The effect of combination chemotherapy on ovarian function in women treated for Hodgkin's disease. Cancer 52: 988–993
Santoro A et al. (1987) Long-term results of combined chemotherapy-radiotherapy approach in Hodgkin's disease: superiority of ABVD plus radiotherapy versus MOPP plus radiotherapy. J Clin Oncol 5: 27–37
Pryzant RM et al. (1993) Long-term reduction in sperm count after chemotherapy with and without radiation therapy for non-Hodgkin's lymphomas. J Clin Oncol 11: 239–247
Dann EJ et al. (2005) Fertility and ovarian function are preserved in women treated with an intensified regimen of cyclophosphamide, adriamycin, vincristine and prednisone (Mega-CHOP) for non-Hodgkin lymphoma. Hum Reprod 20: 2247–2249
Wallace WH et al. (1991) Male fertility in long-term survivors of childhood acute lymphoblastic leukaemia. Int J Androl 14: 312–319
Wallace WH et al. (1993) Ovarian function following the treatment of childhood acute lymphoblastic leukaemia. Med Pediatr Oncol 21: 333–339
Waxman J et al. (1983) Gonadal function in men treated for acute leukaemia. Br Med J (Clin Res Ed) 287: 1093–1094
Leung W et al. (2000) Late effects of treatment in survivors of childhood acute myeloid leukemia. J Clin Oncol 18: 3273–3279
Chemaitilly W et al. (2006) Acute ovarian failure in the childhood cancer survivor study. J Clin Endocrinol Metab 91: 1723–1728
Spinelli S et al. (1994) Ovarian recovery after total body irradiation and allogeneic bone marrow transplantation: long-term follow up of 79 females. Bone Marrow Transplant 14: 373–380
Schimmer AD et al. (1998) Ovarian function after autologous bone marrow transplantation. J Clin Oncol 16: 2359–2363
Sanders JE (2004) Endocrine complications of high-dose therapy with stem cell transplantation. Pediatr Transplant 8 (Suppl 5): 39–50
Heimpel H et al. (1991) Gonadal function after bone marrow transplantation in adult male and female patients. Bone Marrow Transplant 8 (Suppl 1): 21–24
Chatterjee R et al. (2001) Patterns of Leydig cell insufficiency in adult males following bone marrow transplantation for haematological malignancies. Bone Marrow Transplant 28: 497–502
Jacob A et al. (1998) Recovery of spermatogenesis following bone marrow transplantation. Bone Marrow Transplant 22: 277–279
Salooja N et al. (2001) Pregnancy outcomes after peripheral blood or bone marrow transplantation: a retrospective survey. Lancet 358: 271–276
Carter A et al. (2006) Prevalence of conception and pregnancy outcomes after hematopoietic cell transplantation: report from the Bone Marrow Transplant Survivor Study. Bone Marrow Transplant 37: 1023–1029
Cheng YC et al. (2005) Low prevalence of premature ovarian failure in women given reduced-intensity conditioning regimens for hematopoietic stem-cell transplantation. Haematologica 90: 1725–1726
Ash P (1980) The influence of radiation on fertility in man. Br J Radiol 53: 271–278
Shalet SM (1993) Effect of irradiation treatment on gonadal function in men treated for germ cell cancer. Eur Urol 23: 148–151
Speiser B et al. (1973) Aspermia following lower truncal irradiation in Hodgkin's disease. Cancer 32: 692–698
Signorello LB et al. (2006) Female survivors of childhood cancer: preterm birth and low birth weight among their children. J Natl Cancer Inst 98: 1453–1461
Critchley HO and Wallace WH (2005) Impact of cancer treatment on uterine function. J Natl Cancer Inst Monogr 64–68
Wallace WH et al. (2005) Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys 62: 738–744
Littley MD et al. (1989) Radiation-induced hypopituitarism is dose-dependent. Clin Endocrinol (Oxf) 31: 363–373
Pai HH et al. (2001) Hypothalamic/pituitary function following high-dose conformal radiotherapy to the base of skull: demonstration of a dose-effect relationship using dose-volume histogram analysis. Int J Radiat Oncol Biol Phys 49: 1079–1092
World Health Organization (ed 4, 1999) Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. Cambridge: Cambridge University Press
Speroff L and Fritz MA (2005) Male infertility. In: Clinical Gynecologic Endocrinology and Infertility 1135. Philadelphia, PA: Lippincott Williams & Wilkins
Chatterjee R and Kottaridis PD (2002) Treatment of gonadal damage in recipients of allogeneic or autologous transplantation for haematological malignancies. Bone Marrow Transplant 30: 629–635
Tanner JM and Whitehouse RH (1976) Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child 51: 170–179
Thomson AB et al. (2002) Late reproductive sequelae following treatment of childhood cancer and options for fertility preservation. Best Pract Res Clin Endocrinol Metab 16: 311–334
Oktay K et al. (2006) Measuring the impact of chemotherapy on fertility in women with breast cancer. J Clin Oncol 24: 4044–4046
Ruess ML et al. (1996) Age and the ovarian follicle pool assessed with transvaginal ultrasonography. Am J Obstet Gynecol 174: 624–627
Blumenfeld Z (2002) Preservation of fertility and ovarian function and minimalization of chemotherapy associated gonadotoxicity and premature ovarian failure: the role of inhibin-A and -B as markers. Mol Cell Endocrinol 187: 93–105
Yong PY et al. (2003) Prospective analysis of the relationships between the ovarian follicle cohort and basal FSH concentration, the inhibin response to exogenous FSH and ovarian follicle number at different stages of the normal menstrual cycle and after pituitary down-regulation. Hum Reprod 18: 35–44
Josso N et al. (1993) Anti-mullerian hormone: the Jost factor. Recent Prog Horm Res 48: 1–59
Bath LE et al. (2003) Depletion of ovarian reserve in young women after treatment for cancer in childhood: detection by anti-Mullerian hormone, inhibin B and ovarian ultrasound. Hum Reprod 18: 2368–2374
Anderson RA et al. (2006) The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod 21: 2583–2592
Thomson AB et al. (2002) Semen quality and spermatozoal DNA integrity in survivors of childhood cancer: a case-control study. Lancet 360: 361–367
Agarwal A et al. (2004) Fertility after cancer: a prospective review of assisted reproductive outcome with banked semen specimens. Fertil Steril 81: 342–348
Meseguer M et al. (2006) Sperm cryopreservation in oncological patients: a 14-year follow-up study. Fertil Steril 85: 640–645
Styer AK et al. (2003) Factors associated with disposition of cryopreserved reproductive tissue. Fertil Steril 80: 584–589
Klemetti R et al. (2005) Children born after assisted fertilization have an increased rate of major congenital anomalies. Fertil Steril 84: 1300–1307
Rimm AA et al. (2004) A meta-analysis of controlled studies comparing major malformation rates in IVF and ICSI infants with naturally conceived children. J Assist Reprod Genet 21: 437–443
Olson CK et al. (2005) In vitro fertilization is associated with an increase in major birth defects. Fertil Steril 84: 1308–1315
Hansen M et al. (2005) Assisted reproductive technologies and the risk of birth defects—a systematic review. Hum Reprod 20: 328–338
Honaramooz A et al. (2002) Sperm from neonatal mammalian testes grafted in mice. Nature 418: 778–781
Jahnukainen K et al. (2001) Intratesticular transplantation of testicular cells from leukemic rats causes transmission of leukemia. Cancer Res 61: 706–710
Ogawa T et al. (2000) Transplantation of male germ line stem cells restores fertility in infertile mice. Nat Med 6: 29–34
Radford J et al. (1999) Fertility after treatment for cancer. Questions remain over ways of preserving ovarian and testicular tissue. BMJ 319: 935–936
Jahnukainen K et al. (2006) Clinical potential and putative risks of fertility preservation in children utilizing gonadal tissue or germline stem cells. Pediatr Res 59: 40R–47R
Wang JX et al. (2001) Frozen-thawed embryo transfer: influence of clinical factors on implantation rate and risk of multiple conception. Hum Reprod 16: 2316–2319
American Society for Reproductive Medicine/Society for Assisted Reproductive Technology Registry (2002) Assisted reproductive technology in the United States: 1998 results generated from the American Society for Reproductive Medicine/Society for Assisted Reproductive Technology Registry. Fertil Steril 77: 18–31
Lipton JH et al. (1997) Successful pregnancy after allogeneic bone marrow transplant with embryos isolated before transplant. J Clin Oncol 15: 3347–3349
Haie-Meder C et al. (1993) Radiotherapy after ovarian transposition: ovarian function and fertility preservation. Int J Radiat Oncol Biol Phys 25: 419–424
Williams RS et al. (1999) Laparoscopic oophoropexy and ovarian function in the treatment of Hodgkin disease. Cancer 86: 2138–2142
Clough KB et al. (1996) Laparoscopic unilateral ovarian transposition prior to irradiation: prospective study of 20 cases. Cancer 77: 2638–2645
Oktay K et al. (2006) Efficiency of oocyte cryopreservation: a meta-analysis. Fertil Steril 86: 70–80
La Sala GB et al. (2006) Outcome of 518 salvage oocyte-cryopreservation cycles performed as a routine procedure in an in vitro fertilization program. Fertil Steril 86: 1423–1427
Sonmezer M and Oktay K (2004) Fertility preservation in female patients. Hum Reprod Update 10: 251–266
Falcone T et al. (2004) Ovarian function preservation in the cancer patient. Fertil Steril 81: 243–257
Donnez J et al. (2004) Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 364: 1405–1410
Meirow D et al. (2005) Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med 353: 318–321
Newton H et al. (1996) Low temperature storage and grafting of human ovarian tissue. Hum Reprod 11: 1487–1491
Shaw JM et al. (1996) Fresh and cryopreserved ovarian tissue samples from donors with lymphoma transmit the cancer to graft recipients. Hum Reprod 11: 1668–1673
Silber SJ and Gosden RG (2007) Ovarian transplantation in a series of monozygotic twins discordant for ovarian failure. N Engl J Med 356: 1382–1384
Ward JA et al. (1990) Protection of spermatogenesis in rats from the cytotoxic procarbazine by the depot formulation of Zoladex, a gonadotropin-releasing hormone agonist. Cancer Res 50: 568–574
Meistrich ML et al. (1999) Hormonal treatment after cytotoxic therapy stimulates recovery of spermatogenesis. Cancer Res 59: 3557–3560
Masala A et al. (1997) Use of testosterone to prevent cyclophosphamide-induced azoospermia. Ann Intern Med 126: 292–295
Johnson DH et al. (1985) Effect of a luteinizing hormone releasing hormone agonist given during combination chemotherapy on posttherapy fertility in male patients with lymphoma: preliminary observations. Blood 65: 832–836
Thomson AB et al. (2002) Investigation of suppression of the hypothalamic-pituitary-gonadal axis to restore spermatogenesis in azoospermic men treated for childhood cancer. Hum Reprod 17: 1715–1723
Blumenfeld Z et al. (1996) Prevention of irreversible chemotherapy-induced ovarian damage in young women with lymphoma by a gonadotrophin-releasing hormone agonist in parallel to chemotherapy. Hum Reprod 11: 1620–1626
Schover LR et al. (2002) Oncologists' attitudes and practices regarding banking sperm before cancer treatment. J Clin Oncol 20: 1890–1897
Saito K et al. (2005) Sperm cryopreservation before cancer chemotherapy helps in the emotional battle against cancer. Cancer 104: 521–524
McQueeney DA et al. (1997) Efficacy of emotion-focused and problem-focused group therapies for women with fertility problems. J Behav Med 20: 313–331
Ethics Committee of the American Society for Reproductive Medicine (2005) Fertility preservation and reproduction in cancer patients. Fertil Steril 83: 1622–1628
British Fertility Society (2003) Multidisciplinary Working Group convened by the British Fertility Society: A strategy for fertility services for survivors of childhood cancer. Hum Fertil (Camb) 6: A1–A39
Acknowledgements
We thank Christine F Wogan and Donald Norwood, Scientific Editors at the Department of Scientific Publications at MD Anderson Cancer Center for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Nakayama, K., Milbourne, A., Schover, L. et al. Gonadal failure after treatment of hematologic malignancies: from recognition to management for health-care providers. Nat Rev Clin Oncol 5, 78–89 (2008). https://doi.org/10.1038/ncponc1016
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncponc1016
This article is cited by
-
The role of HIF-1α-mediated autophagy in ionizing radiation-induced testicular injury
Journal of Molecular Histology (2023)
-
Fertility Management for the Hemato-Oncologist
Indian Journal of Hematology and Blood Transfusion (2018)
-
Carvedilol alleviates testicular and spermatological damage induced by cisplatin in rats via modulation of oxidative stress and inflammation
Archives of Pharmacal Research (2016)
-
Primordial Follicle Transplantation within Designer Biomaterial Grafts Produce Live Births in a Mouse Infertility Model
Scientific Reports (2015)
-
Successful pregnancy in a seminoma patient after fertility preservation
The Chinese-German Journal of Clinical Oncology (2012)