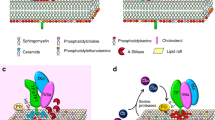
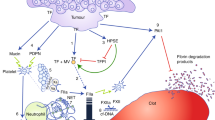
Abstract
Venous thromboembolism is a common complication in patients with malignant disease. It is associated with a systemic hypercoagulable state that is secondary to tumor elaboration of tissue factor (the physiological initiator of blood coagulation), activation of other procoagulant mechanisms and downregulation of anticoagulant mechanisms. The consequent generation of activated coagulation serine protease in the peritumoral environment influences tumor growth, invasion, metastasis and angiogenesis. The use of antithrombotic agents, such as the low-molecular-weight heparins, might influence survival in cancer patients through several mechanisms. These mechanisms include a reduction in the frequency of overt and silent fatal thromboembolic events, interference with the activation of blood coagulation and generation of coagulation serine proteases that affect the tumor phenotype, and direct cellular effects of heparin on both epithelial and endothelial tumor elements.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Trousseau A (1865) Phlegmasia alba dolens. In Clinique Medicale de l'Hotel Dieu de Paris edn 2 (3 vols) 654–712 Paris: Ballière
Geerts WH et al. (2004) Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126 (Suppl): 338S–400S
Buller HR et al. (2004) Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126 (Suppl): 401S–428S
Prandoni P et al. (2002) Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 100: 3484–3488
Lee AY (2005) Management of thrombosis in cancer: primary prevention and secondary prophylaxis. Br J Haematol 128: 291–302
Kakkar AK et al. (1995) Extrinsic-pathway activation in cancer with high factor VIIa and tissue factor. Lancet 346: 1004–1005
Dahlback B (2000) Blood coagulation. Lancet 355: 1627–1632
Virchow R (1856) Collected essays on scientific medicine. (Gesammelte abhandlungen zur wissenschaftlichen medizin) 219–732 Frankfurt: Meidinger
Ott I et al. (1998) A role for tissue factor in cell adhesion and migration mediated by interaction with actin-binding protein 280. J Cell Biol 140: 1241–1253
Kakkar AK et al. (1995) Tissue factor expression correlates with histological grade in human pancreatic cancer. Br J Surg 82: 1101–1104
Rickles FR et al. (1995) Tissue factor expression in human leukocytes and tumor cells. Thromb Haemost 74: 391–395
Tallman MS and Kwaan HC (1992) Reassessing the hemostatic disorder associated with acute promyelocytic leukemia. Blood 79: 543–553
Donati MB et al. (1986) Cancer procoagulant in human tumor cells: evidence from melanoma patients. Cancer Res 46: 6471–6474
Kakkar AK et al. (1999) Role of tissue factor expression on tumour cell invasion and growth of experimental pancreatic adenocarcinoma. Br J Surg 86: 890–894
Zhang Y et al. (1994) Tissue factor controls the balance of angiogenic and antiangiogenic properties of tumor cells in mice. J Clin Invest 94: 1320–1327
Contrino J et al. (1996) In situ detection of tissue factor in vascular endothelial cells: correlation with the malignant phenotype of human breast disease. Nature Med 2: 209–215
Poon RT et al. (2003) Tissue factor expression correlates with tumor angiogenesis and invasiveness in human hepatocellular carcinoma. Clin Cancer Res 9: 5339–5345
Dorfleutner A et al. (2004) Cross-talk of integrin alpha3beta1 and tissue factor in cell migration. Mol Biol Cell 15: 4416–4425
Carmeliet P et al. (1996) Role of tissue factor in embryonic blood vessel development. Nature 383: 73–75
Belting M et al. (2004) Regulation of angiogenesis by tissue factor cytoplasmic domain signaling. Nature Med 10: 502–509
Beerepoot LV et al. (2004) Circulating endothelial cells in cancer patients do not express tissue factor. Cancer Lett 213: 241–248
Rickles FR et al. (2001) The role of the hemostatic system in tumor growth, metastasis, and angiogenesis: tissue factor is a bifunctional molecule capable of inducing both fibrin deposition and angiogenesis in cancer. Int J Hematol 72: 145–150
Shweiki D et al. (1992) Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359: 843–845
Poon RT et al. (2001) Clinical implications of circulating angiogenic factors in cancer patients. J Clin Oncol 19: 1207–1225
Mohle R et al. (1997) Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci USA 94: 663–668
Wang X et al. (2004) Downregulation of tissue factor by RNA interference in human melanoma LOX-L cells reduces pulmonary metastasis in nude mice. Int J Cancer 112: 994–1002
Versteeg HH et al. (2004) Coagulation factors VIIa and Xa inhibit apoptosis and anoikis. Oncogene 23: 410–417
Yu JL et al. (2005) Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood 105: 1734–1741
Even-Ram S et al. (1998) Thrombin receptor overexpression in malignant and physiological invasion processes. Nature Med 4: 909–914
Levine MN et al. (2003) From Trousseau to targeted therapy: new insights and innovations in thrombosis and cancer. J Thromb Haemost 1: 1456–1463
Taniguchi T et al. (1998) Enhanced expression of urokinase receptor induced through the tissue factor–factor VIIa pathway in human pancreatic cancer. Cancer Res 58: 4461–4467
Wojtukiewicz MZ et al. (2001) Localization of blood coagulation factors in situ in pancreatic carcinoma. Thromb Haemost 86: 1416–1420
Caunt M et al. (2003) Thrombin induces neoangiogenesis in the chick chorioallantoic membrane. J Thromb Haemost 1: 2097–2102
Zacharski LR et al. (1984) Effect of warfarin anticoagulation on survival in carcinoma of the lung, colon, head and neck, and prostate. Final report of VA Cooperative Study #75. Cancer 53: 2046–2052
Lebeau B et al. (1994) Subcutaneous heparin treatment increases survival in small cell lung cancer. “Petites Cellules” Group. Cancer 74: 38–45
Gould MK et al. (1999) Low-molecular-weight heparins compared with unfractionated heparin for treatment of acute deep venous thrombosis. A meta-analysis of randomized, controlled trials. Ann Intern Med 130: 800–809
Kakkar AK et al. (2004) Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: the fragmin advanced malignancy outcome study (FAMOUS). J Clin Oncol 22: 1944–1948
Lee AY et al. (2003) Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 349: 146–153
Lee AY et al. (2005) Randomized comparison of low molecular weight heparin and coumarin derivatives on the survival of outpatients with cancer and venous thromboembolism. J Clin Oncol 23: 2123–2129
Altinbas M et al. (2004) A randomized clinical trial of combination chemotherapy with and without low-molecular-weight heparin in small cell lung cancer. J Thromb Haemost 2: 1266–1271
Klerk CP et al. (2005) The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol 23: 2130–2135
Smorenburg SM and Van Noorden CJ (2001) The complex effects of heparins on cancer progression and metastasis in experimental studies. Pharmacol Rev 53: 93–105
Vlodavsky I et al. (1999) Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis. Nature Med 5: 793–802
Borsig L et al. (2001) Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc Natl Acad Sci USA 98: 3352–3357
Yoshitomi Y et al. (2004) Inhibition of experimental lung metastases of Lewis lung carcinoma cells by chemically modified heparin with reduced anticoagulant activity. Cancer Lett 207: 165–174
Da Silva MS et al. (2003) Heparin modulates integrin-mediated cellular adhesion: specificity of interactions with alpha and beta integrin subunits. Cell Commun Adhes 10: 59–67
Mousa SA and Mohamed S (2004) Anti-angiogenic mechanisms and efficacy of the low molecular weight heparin, tinzaparin: anti-cancer efficacy. Oncol Rep 12: 683–688
Mousa SA and Mohamed S (2004) Inhibition of endothelial cell tube formation by the low molecular weight heparin, tinzaparin, is mediated by tissue factor pathway inhibitor. Thromb Haemost 92: 627–633
Folkman J et al. (1983) Angiogenesis inhibition and tumor regression caused by heparin or a heparin fragment in the presence of cortisone. Science 221: 719–725
Nasir FA et al. (2003) The low molecular weight heparin dalteparin sodium inhibits angiogenesis and induces apoptosis in an experimental tumour model. Blood 102: [Abstract #2993]
Samoszuk M et al. (2003) Inhibition of thrombosis in melanoma allografts in mice by endogenous mast cell heparin. Thromb Haemost 90: 351–360
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- SUPERFICIAL THROMBOPHLEBITIS
-
A condition in which a blood clot forms in a vein near the surface of the body as a result of inflammation
- MONOCYTES
-
Phagocytic white blood cells that can develop into macrophages following migration into tissue
- MEGAKARYOCYTE
-
A giant bone-marrow cell that generates platelets
- THROMBOCYTHEMIA
-
A myeloproliferative disorder that is characterized by overproduction of platelets in the bone marrow
- SHORT HAIRPIN RNA
-
A short sequence of RNA that forms a hairpin structure and can be used to silence gene expression at the transcriptional level
- RNA INTERFERENCE
-
Use of double-stranded RNA to interfere with normal RNA processing, causing rapid degradation of endogenous RNA and precluding translation; a simple method of studying the effect of absence of a gene product
- NUDE MICE
-
Hairless mice homozygous for a recessive mutation of the nu gene; they have no thymus and cannot generate mature T lymphocytes
- G PROTEIN
-
GTP-binding regulatory proteins in the cell membrane, which act as intermediaries between cell-surface receptors and intracellular enzymes, enabling regulation of enzyme activity in response to extracellular signals
- CHORIOALLANTOIC MEMBRANE
-
A chick extraembryonic membrane that is often used as an in vivo model to study the formation of new blood vessels
- INTERNATIONAL NORMALIZED RATIO
-
A method used to compare the activity of different batches of thromboplastin
- HAZARD RATIO (HR)
-
The relative likelihood of experiencing a particular event; an HR of 0.5 indicates that one group has half the risk of the other group
Rights and permissions
About this article
Cite this article
Petralia, G., Lemoine, N. & Kakkar, A. Mechanisms of Disease: the impact of antithrombotic therapy in cancer patients. Nat Rev Clin Oncol 2, 356–363 (2005). https://doi.org/10.1038/ncponc0225
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncponc0225
This article is cited by
-
Pathologies at the nexus of blood coagulation and inflammation: thrombin in hemostasis, cancer, and beyond
Journal of Molecular Medicine (2013)
-
Thrombosis and cancer
Nature Reviews Clinical Oncology (2012)
-
Antithrombotic therapy and survival in patients with malignant disease
British Journal of Cancer (2010)