Abstract
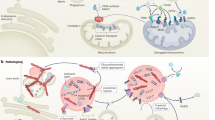
Parkinson disease (PD) is associated with progressive loss of dopaminergic neurons in the substantia nigra, as well as with more-widespread neuronal changes that cause complex and variable motor and nonmotor symptoms. Recent rapid advances in PD genetics have revealed a prominent role for mitochondrial dysfunction in the pathogenesis of the disease, and the products of several PD-associated genes, including SNCA, Parkin, PINK1, DJ-1, LRRK2 and HTR2A, show a degree of localization to the mitochondria under certain conditions. Impaired mitochondrial function is likely to increase oxidative stress and might render cells more vulnerable to this and other related processes, including excitotoxicity. The mitochondria, therefore, represent a highly promising target for the development of disease biomarkers by use of genetic, biochemical and bioimaging approaches. Novel therapeutic interventions that modify mitochondrial function are currently under development, and a large phase III clinical trial is underway to examine whether high-dose oral coenzyme Q10 will slow disease progression. In this Review, we examine evidence for the roles of mitochondrial dysfunction and increased oxidative stress in the neuronal loss that leads to PD and discuss how this knowledge might further improve patient management and aid in the development of 'mitochondrial therapy' for PD.
Key Points
-
Defective mitochondrial function and increased oxidative stress have been demonstrated in a subset of people with Parkinson disease (PD)
-
The products of several nuclear genes associated with PD are linked to mitochondrial function
-
Mitochondrial activity can also be affected by environmental factors that possibly contribute to PD pathogenesis
-
Novel therapies that target mitochondrial function and oxidative stress, such as coenzyme Q10, are now in clinical trials to test whether they modify PD progression
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Beal MF (2007) Mitochondria and neurodegeneration. Novartis Found Symp 287: 183–192
Schapira AH (2008) Mitochondria in the aetiology and pathogenesis of Parkinson's disease. Lancet Neurol 7: 97–109
Schapira AH et al. (1990) Mitochondrial complex I deficiency in Parkinson's disease. J Neurochem 54: 823–827
Parker WD Jr et al. (2008) Complex I deficiency in Parkinson's disease frontal cortex. Brain Res 1189: 215–218
Keeney PM et al. (2006) Parkinson's disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J Neurosci 26: 5256–5264
Haas RH et al. (1995) Low platelet mitochondrial complex I and complex II/III activity in early untreated Parkinson's disease. Ann Neurol 37: 714–722
Penn AM et al. (1995) Generalized mitochondrial dysfunction in Parkinson's disease detected by magnetic resonance spectroscopy of muscle. Neurology 45: 2097–2099
Swerdlow RH et al. (1996) Origin and functional consequences of the complex I defect in Parkinson's disease. Ann Neurol 40: 663–671
Gu M et al. (1998) Mitochondrial DNA transmission of the mitochondrial defect in Parkinson's disease. Ann Neurol 44: 177–186
Hu MT et al. (2000) Cortical dysfunction in non-demented Parkinson's disease patients: a combined 31P-MRS and 18FDG-PET study. Brain 123: 340–352
Rango M et al. (2005) Parkinson's disease and brain mitochondrial dysfunction: a functional phosphorus magnetic resonance spectroscopy study. J Cereb Blood Flow Metab 26: 283–290
Bowen BC et al. (1995) Proton MR spectroscopy of the brain in 14 patients with Parkinson disease. AJNR Am J Neuroradiol 16: 61–68
Henchcliffe C et al.: Multinuclear magnetic resonance spectroscopy for in vivo assessment of mitochondrial function in Parkinson's disease. Ann N Y Acad Sci, in press
Dexter DT et al. (1989) Basal lipid peroxidation in substantia nigra is increased in Parkinson's disease. J Neurochem 52: 381–389
Zhang J et al. (1999) Parkinson's disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am J Pathol 154: 1423–1429
Perry TL et al. (1986) Idiopathic Parkinson's disease, progressive supranuclear palsy and glutathione metabolism in the substantia nigra of patients. Neurosci Lett 67: 269–274
Jenner P (2003) Oxidative stress in Parkinson's disease. Ann Neurol 53 (Suppl 3): S26–S36
Bogdanov M et al. (2008) Metabolomic profiling to develop blood biomarkers for Parkinson's disease. Brain 131: 389–396
Weisskopf MG et al. (2007) Plasma urate and risk of Parkinson's disease. Am J Epidemiol 166: 561–567
Green DR et al. (2004) The pathophysiology of mitochondrial cell death. Science 305: 626–629
Beal MF (1998) Excitotoxicity and nitric oxide in Parkinson's disease pathogenesis. Ann Neurol 44 (Suppl 1): S110–S114
Langston JW et al. (1983) Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219: 979–980
Betarbet R et al. (2000) Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci 3: 1301–1306
Coulom H et al. (2004) Chronic exposure to rotenone models sporadic Parkinson's disease in Drosophila melanogaster. J Neurosci 24: 10993–10998
Cicchetti F et al. (2005) Systemic exposure to paraquat and maneb models early Parkinson's disease in young adult rats. Neurobiol Dis 20: 360–371
Greenamyre JT et al. (1994) Antiparkinsonian effects of remacemide hydrochloride, a glutamate antagonist, in rodent and primate models of Parkinson's disease. Ann Neurol 35: 655–661
Swerdlow RH et al. (1998) Matrilineal inheritance of complex I dysfunction in a multigenerational Parkinson's disease family. Ann Neurol 44: 873–881
Wooten GF et al. (1997) Maternal inheritance in Parkinson's disease. Ann Neurol 41: 265–268
Simon DK et al. (1999) Familial multisystem degeneration with parkinsonism associated with the 11778 mitochondrial DNA mutation. Neurology 53: 1787–1793
Luoma P et al. (2004) Parkinsonism, premature menopause, and mitochondrial DNA polymerase gamma mutations: clinical and molecular genetic study. Lancet 364: 875–882
Bender A et al. (2006) High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet 38: 515–517
Srivastava S et al. (2005) Double-strand breaks of mouse muscle mtDNA promote large deletions similar to multiple mtDNA deletions in humans. Hum Mol Genet 14: 893–902
Pyle A et al. (2005) Mitochondrial DNA haplogroup cluster UKJT reduces the risk of PD. Ann Neurol 57: 564–567
Devi L et al. (2008) Mitochondrial import and accumulation of α-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem 283: 9089–9100
Cole NB et al. (2008) Mitochondrial translocation of alpha-synuclein is promoted by intracellular acidification. Exp Cell Res 314: 2076–2089
Shavali S et al. (2008) Mitochondrial localization of alpha-synuclein protein in alpha-synuclein overexpressing cells. Neurosci Lett 439: 125–128
Martin LJ et al. (2006) Parkinson's disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J Neurosci 26: 41–50
Parihar MS et al. (2008) Mitochondrial association of alpha-synuclein causes oxidative stress. Cell Mol Life Sci 65: 1272–1284
Buttner S et al. (2008) Functional mitochondria are required for α-synuclein toxicity in aging yeast. J Biol Chem 283: 7554–7560
Dauer W et al. (2003) Parkinson's disease: mechanisms and models. Neuron 39: 889–909
Song DD et al. (2004) Enhanced substantia nigra mitochondrial pathology in human α-synuclein transgenic mice after treatment with MPTP. Exp Neurol 186: 158–172
Kuroda Y et al. (2006) Parkin enhances mitochondrial biogenesis in proliferating cells. Hum Mol Genet 15: 883–895
Greene JC et al. (2003) Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci USA 100: 4078–4083
Pesah Y et al. (2004) Drosophila parkin mutants have decreased mass and cell size and increased sensitivity to oxygen radical stress. Development 131: 2183–2194
Palacino JJ et al. (2004) Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J Biol Chem 279: 18614–18622
Muftuoglu M et al. (2004) Mitochondrial complex I and IV activities in leukocytes from patients with parkin mutations. Mov Disord 19: 544–548
Poole AC et al. (2008) The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci USA 105: 1638–1643
Silvestri L et al. (2005) Mitochondrial import and enzymatic activity of PINK1 mutants associated to recessive parkinsonism. Hum Mol Genet 14: 3477–3492
Pridgeon JW et al. (2007) PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol 5: e172
Hoepken HH et al. (2007) Mitochondrial dysfunction, peroxidation damage and changes in glutathione metabolism in PARK6. Neurobiol Dis 25: 401–411
Clark IE et al. (2006) Drosophila Pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 441: 1162–1166
Yang Y et al. (2008) Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci USA 105: 7070–7075
Petit A et al. (2005) Wild-type PINK1 prevents basal and induced neuronal apoptosis, a protective effect abrogated by Parkinson disease-related mutations. J Biol Chem 280: 34025–34032
Wood-Kaczmar A et al. (2008) PINK1 is necessary for long term survival and mitochondrial function in human dopaminergic neurons. PLoS ONE 3: e2455
Exner N et al. (2007) Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J Neurosci 27: 12413–12418
Bonifati V et al. (2003) Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299: 256–259
Yokota T et al. (2003) Down regulation of DJ-1 enhances cell death by oxidative stress, ER stress, and proteasome inhibition. Biochem Biophys Res Commun 312: 1342–1348
Zhang L et al. (2005) Mitochondrial localization of the Parkinson's disease related protein DJ-1: implications for pathogenesis. Hum Mol Genet 14: 2063–2073
Canet-Aviles RM et al. (2004) The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci USA 101: 9103–9108
Meulener M et al. (2005) Drosophila DJ-1 mutants are selectively sensitive to environmental toxins associated with Parkinson's disease. Curr Biol 15: 1572–1577
Park J et al. (2005) Drosophila DJ-1 mutants show oxidative stress-sensitive locomotive dysfunction. Gene 361: 133–139
Yang Y et al. (2005) Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc Natl Acad Sci USA 102: 13670–13675
Shendelman S et al. (2004) DJ-1 is a redox-dependent molecular chaperone that inhibits α-synuclein aggregate formation. PLoS Biol 2: e362
Meulener MC et al. (2005) DJ-1 is present in a large molecular complex in human brain tissue and interacts with α-synuclein. J Neurochem 93: 1524–1532
West AB et al. (2005) Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci USA 102: 16842–16847
Martins LM et al. (2004) Neuroprotective role of the Reaper-related serine protease HtrA2/Omi revealed by targeted deletion in mice. Mol Cell Biol 24: 9848–9862
Strauss KM et al. (2005) Loss of function mutations in the gene encoding Omi/HtrA2 in Parkinson's disease. Hum Mol Genet 14: 2099–2111
Ravagnan L et al. (2002) Mitochondria, the killer organelles and their weapons. J Cell Physiol 192: 131–137
Plun-Favreau H et al. (2007) The mitochondrial protease HtrA2 is regulated by Parkinson's disease-associated kinase PINK1. Nat Cell Biol 9: 1243–1252
Simon-Sanchez J et al. (2008) Sequencing analysis of OMI/HTRA2 shows previously reported pathogenic mutations in neurologically normal controls. Hum Mol Genet 17: 1988–1993
Beal MF (2004) Mitochondrial dysfunction and oxidative damage in Alzheimer's and Parkinson's diseases and coenzyme Q10 as a potential treatment. J Bioenerg Biomembr 36: 381–386
McCarthy S et al. (2004) Paraquat induces oxidative stress and neuronal cell death; neuroprotection by water-soluble coenzyme Q10. Toxicol Appl Pharmacol 201: 21–31
Menke T et al. (2003) Coenzyme Q10 reduces the toxicity of rotenone in neuronal cultures by preserving the mitochondrial membrane potential. Biofactors 18: 65–72
Beal MF et al. (1998) Coenzyme Q10 attenuates the 1-methyl-4-phenyl-1,2,3,tetrahydropyridine (MPTP) induced loss of striatal dopamine and dopaminergic axons in aged mice. Brain Res 783: 109–114
Horvath TL et al. (2003) Coenzyme Q induces nigral mitochondrial uncoupling and prevents nigral cell loss in a primate model of Parkinson's disease. Endocrinology 144: 2757–2760
Stamelou M et al. (2008) Short-term effects of coenzyme Q10 in progressive supranuclear palsy: a randomized, placebo-controlled trial. Mov Disord 23: 942–949
Shults CW et al. (2002) Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol 59: 1541–1550
The NINDS NET-PD Investigators (2007) A randomized clinical trial of coenzyme Q10 and GPI-1485 in early Parkinson disease. Neurology 68: 20–28
The NINDS NET-PD Investigators (2006) A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology 66: 664–671
Klivenyi P et al. (2006) Mice lacking alpha-synuclein are resistant to mitochondrial toxins. Neurobiol Dis 21: 541–548
Parkinson Study Group (2004) A controlled, randomized, delayed-start study of rasagiline in early Parkinson disease. Arch Neurol 61: 561–566
Lagouge M et al. (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 127: 1109–1122
Lu KT et al. (2008) Neuroprotective effects of resveratrol on MPTP-induced neuron loss mediated by free radical scavenging. J Agric Food Chem 56: 6910–6913
Ko L et al. (2000) Sensitization of neuronal cells to oxidative stress with mutated human α-synuclein. J Neurochem 75: 2546–2554
Ostrerova-Golts N et al. (2000) The A53T α-synuclein mutation increases iron-dependent aggregation and toxicity. J Neurosci 20: 6048–6054
Smith WW et al. (2005) Leucine-rich repeat kinase 2 (LRRK2) interacts with parkin, and mutant LRRK2 induces neuronal degeneration. Proc Natl Acad Sci USA 102: 18676–18681
Paxinou E et al. (2001) Induction of α-synuclein aggregation by intracellular nitrative insult. J Neurosci 21: 8053–8061
Darios F et al. (2003) Parkin prevents mitochondrial swelling and cytochrome c release in mitochondria-dependent cell death. Hum Mol Genet 12: 517–526
Park J et al. (2006) Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 441: 1157–1161
Gandhi S et al. (2006) PINK1 protein in normal human brain and Parkinson's disease. Brain 129: 1720–1731
Taira T et al. (2004) DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep 5: 213–218
Choi J et al. (2006) Oxidative damage of DJ-1 is linked to sporadic Parkinson and Alzheimer diseases. J Biol Chem 281: 10816–10824
Menzies FM et al. (2005) Roles of Drosophila DJ-1 in survival of dopaminergic neurons and oxidative stress. Curr Biol 15: 1578–1582
Acknowledgements
The authors would like to thank Ms Greta Strong for her outstanding assistance, and Penelope Grossman M.D. for helpful comments on the manuscript. CH acknowledges support of the Daisy and Paul Soros Clinical Scholarship in Neurology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Henchcliffe, C., Beal, M. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Rev Neurol 4, 600–609 (2008). https://doi.org/10.1038/ncpneuro0924
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpneuro0924
This article is cited by
-
No causal relationship between thyroid function and Parkinson’s disease: A bidirectional Mendelian randomization study
Neurological Sciences (2024)
-
Translational molecular imaging and drug development in Parkinson’s disease
Molecular Neurodegeneration (2023)
-
A beginner’s guide into curated analyses of open access datasets for biomarker discovery in neurodegeneration
Scientific Data (2023)
-
New insights on the potential effect of vinpocetine in Parkinson’s disease: one of the neglected warden and baffling topics
Metabolic Brain Disease (2023)
-
Folic Acid and Folinic Acid Protect Hearts of Aging Triple-transgenic Alzheimer’s Disease mice via IGF1R/PI3K/AKT and SIRT1/AMPK Pathways
Neurotoxicity Research (2023)