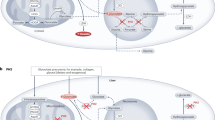
Abstract
Progressive loss of kidney function complicates Fabry disease, an X-linked lysosomal storage disorder that arises from deficiency of α-galactosidase activity. Heterozygous females with Fabry disease can be as severely affected as hemizygous males, who have the classic form of the disease. Enzyme-replacement therapy with recombinant human α-galactosidase clears the glycosphingolipid globotriaosylceramide from kidney cells, and can stabilize renal function in adults with mild to moderate Fabry nephropathy. However, adults with more advanced nephropathy and overt proteinuria do not respond as well. For these patients, antiproteinuric therapy given in conjunction with enzyme-replacement therapy might prevent further decline in kidney function. In this Review, we propose guidelines and recommendations for the diagnosis and management of Fabry nephropathy in adults, based on published data and on the consensus of opinion of participants in the 7th International Fabry Nephropathy Roundtable in 2007. These organ-specific guidelines could be easier to implement than general guidelines, provided they are used in the context of an overall multisystem care approach.
Key Points
-
Fabry nephropathy is an important cause of morbidity and mortality in males with classic disease, as well as in an appreciable number of females
-
Without suitable screening, Fabry nephropathy can be overlooked; kidney biopsy can confirm the diagnosis, stage the severity of the disease, and gauge the response to therapy
-
Enzyme-replacement therapy (ERT) clears globotriaosylceramide from many kidney cell types, and long-term ERT stabilizes renal function in adults with mild to moderate renal impairment, but it does not decrease proteinuria; patients with more advanced nephropathy respond less well
-
ERT should be started as early as possible to delay further organ damage, especially in males with no residual enzyme activity
-
Proteinuria is an important therapeutic target; adjunctive treatment with angiotensin-converting-enzyme inhibitors and/or angiotensin-receptor blockers should be used to reduce urinary total protein excretion to less than 500 mg/day
-
Further studies are needed to define the optimal diagnostic, follow-up and treatment protocols for Fabry nephropathy and to provide an evidence base for current and future guidelines in adults and children
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Desnick R et al. (2001) Alpha-Galactosidase A deficiency: Fabry disease. In The Metabolic Bases of Inherited Disease, edn 8, 3733–3774 (Eds Scriver C et al.) New York: McGraw-Hill
Eng CM et al. (2007) Fabry disease: baseline medical characteristics of a cohort of 1765 males and females in the Fabry Registry. J Inherit Metab Dis 30: 184–192
Mehta A et al. (2004) Fabry disease defined: baseline clinical manifestations of 366 patients in the Fabry Outcome Survey. Eur J Clin Invest 34: 236–242
Wang RY et al. (2007) Heterozygous Fabry women are not just carriers, but have a significant burden of disease and impaired quality of life. Genet Med 9: 34–45
Wilcox WR et al. (2008) Females with X-linked Fabry disease frequently have significant organ involvement. Mol Genet Metab 93: 112–128
Branton MH et al. (2002) Natural history of Fabry renal disease: influence of alpha-galactosidase A activity and genetic mutations on clinical course. Medicine (Baltimore) 81: 122–138
Eng CM et al. (2001) A phase 1/2 clinical trial of enzyme replacement in Fabry disease: pharmacokinetic, substrate clearance, and safety studies. Am J Hum Genet 68: 711–722
Wilcox WR et al. (2004) Long-term safety and efficacy of enzyme replacement therapy for Fabry disease. Am J Hum Genet 75: 65–74
Banikazemi M et al. (2007) Agalsidase-beta therapy for advanced Fabry disease: a randomized trial. Ann Intern Med 146: 77–86
Germain D et al. (2007) Sustained, long-term renal stabilization after 54 months of agalsidase beta therapy in patients with Fabry disease. J Am Soc Nephrol 18: 1547–1557
Desnick RJ (2004) Enzyme replacement therapy for Fabry disease: lessons from two alpha-galactosidase A orphan products and one FDA approval. Expert Opin Biol Ther 4: 1167–1176
Schiffmann R et al. (2001) Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA 285: 2743–2749
Schiffmann R et al. (2006) Long-term therapy with agalsidase alfa for Fabry disease: safety and effects on renal function in a home infusion setting. Nephrol Dial Transplant 21: 345–354
Schiffmann R et al. (2007) Weekly enzyme replacement therapy may slow decline of renal function in Fabry patients who are on long-term biweekly dosing. J Am Soc Nephrol 18: 1576–1583
Ortiz A et al. (2008) Nephropathy in males and females with Fabry disease: cross-sectional description of patients before treatment with enzyme replacement therapy. Nephrol Dial Transplant [10.1093/ndt/gfm848]
Brady RO et al. (1971) Fabry's disease: antenatal detection. Science 172: 174–175
Vedder AC et al. (2006) Manifestations of Fabry disease in placental tissue. J Inherit Metab Dis 29: 106–111
Ries M et al. (2004) Parapelvic kidney cysts: a distinguishing feature with high prevalence in Fabry disease. Kidney Int 66: 978–982
Ries M et al. (2003) The early clinical phenotype of Fabry disease: a study on 35 European children and adolescents. Eur J Pediatr 162: 767–772
Ries M et al. (2005) Pediatric Fabry disease. Pediatrics 115: e344–e355
Gubler MC et al. (1978) Early renal changes in hemizygous and heterozygous patients with Fabry's disease. Kidney Int 13: 223–235
MacDermot KD et al. (2001) Natural history of Fabry disease in affected males and obligate carrier females. J Inherit Metab Dis 24 (Suppl 2): 13–14
Deegan PB et al. (2006) Natural history of Fabry disease in females in the Fabry Outcome Survey. J Med Genet 43: 347–352
Tsakiris D et al. (1996) Report on management of renal failure in Europe, XXVI, 1995. Rare diseases in renal replacement therapy in the ERA-EDTA Registry. Nephrol Dial Transplant 11 (Suppl 7): 4–20
Desnick RJ et al. (2003) Fabry disease, an under-recognized multisystemic disorder: expert recommendations for diagnosis, management, and enzyme replacement therapy. Ann Intern Med 138: 338–346
Hughes DA et al. (online 1 August 2005) Guidelines for the diagnosis and management of Anderson–Fabry Disease [http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/Publications PolicyAndGuidance/DH_4118404] (accessed 23 October 2007)
Clarke LA et al. (online November 2005) Fabry Disease: recommendations for diagnosis, management, and enzyme replacement therapy in Canada [http://www.garrod.ca/data/attachments/CanadianFabryGuidelinesNov05.pdf] (accessed 23 October 2007)
Eng CM et al. (2006) Fabry disease: guidelines for the evaluation and management of multi-organ system involvement. Genet Med 8: 539–548
Fabry Registry (online 2007) Patient Monitoring [https://www.lsdregistry.net/fabryregistry/hcp/partic/freg_hc_p_monitor.asp] (accessed 23 October 2007)
Stevens LA et al. (2006) Assessing kidney function–measured and estimated glomerular filtration rate. N Engl J Med 354: 2473–2483
National Kidney Foundation (2002) K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39 (Suppl 1): S1–S266
Kleinert J et al. (2005) Measurement of renal function in patients with Fabry disease. Acta Paediatr Suppl 94: 19–23
Alroy J et al. (2002) Renal pathology in Fabry disease. J Am Soc Nephrol 13 (Suppl 2): S134–S138
Albay D et al. (2005) Chloroquine-induced lipidosis mimicking Fabry disease. Mod Pathol 18: 733–738
Fischer EG et al. (2006) Fabry disease: a morphologic study of 11 cases. Mod Pathol 19: 1295–1301
de Zeeuw D et al. (2004) Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int 65: 2309–2320
Kent DM et al. (2007) Progression risk, urinary protein excretion, and treatment effects of Angiotensin-converting enzyme inhibitors in nondiabetic kidney disease. J Am Soc Nephrol 18: 1959–1965
Palmer BF (2007) Proteinuria as a therapeutic target in patients with chronic kidney disease. Am J Nephrol 27: 287–293
Thurberg BL et al. (2002) Globotriaosylceramide accumulation in the Fabry kidney is cleared from multiple cell types after enzyme replacement therapy. Kidney Int 62: 1933–1946
Eng CM et al. (2001) Safety and efficacy of recombinant human alpha-galactosidase A-replacement therapy in Fabry's disease. N Engl J Med 345: 9–16
Schwarting A et al. (2006) Enzyme replacement therapy and renal function in 201 patients with Fabry disease. Clin Nephrol 66: 77–84
ClinicalTrials.gov (online April 2007) Canadian Fabry Disease Initiative (CFDI) Enzyme Replacement Therapy (ERT) Study (NCT00455104) [http://clinicaltrials.gov/ct/show/NCT00455104?order=2term=NCT00446862& submit=Search] (accessed 23 October 2007)
Vedder AC et al. (2007) Treatment of Fabry disease: outcome of a comparative trial with agalsidase alfa or beta at a dose of 0.2 mg/kg. PLoS ONE 2: e598
Warnock DG and West ML (2006) Diagnosis and management of kidney involvement in Fabry disease. Adv Chronic Kidney Dis 13: 138–147
Warnock DG (2005) Fabry disease: diagnosis and management, with emphasis on the renal manifestations. Curr Opin Nephrol Hypertens 14: 87–95
Beck M et al. (2004) Fabry disease: overall effects of agalsidase alfa treatment. Eur J Clin Invest 34: 838–844
Warnock DG (2007) Enzyme replacement therapy and Fabry kidney disease: quo vadis? J Am Soc Nephrol 18: 1368–1370
Chobanian AV et al. (2003) The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 289: 2560–2572
Kleinert J et al. (2006) Prevalence of uncontrolled hypertension in patients with Fabry disease. Am J Hypertens 19: 782–787
Tahir H et al. (2007) Antiproteinuric therapy and Fabry nephropathy: sustained reduction of proteinuria in patients receiving enzyme replacement therapy with agalsidase-beta. J Am Soc Nephrol 18: 2609–2617
ClinicalTrials.gov (online 10 April 2007) The Fabrazyme® and Arbs and ACE Inhibitor Treatment (FAACET) Study (NCT00446862) [http://www.clinicaltrials.gov/ct/show/NCT00446862?order=1] (accessed 23 October 2007)
Tondel C et al: Glomerular and vascular changes are prominent in renal biopsies in children and adolescents with Fabry disease and minimal albuminuria. Am J Kidney Dis, in press
Breunig F et al. (2006) Clinical benefit of enzyme replacement therapy in Fabry disease. Kidney Int 69: 1216–1221
Ojo A et al. (2000) Excellent outcome of renal transplantation in patients with Fabry's disease. Transplantation 69: 2337–2339
Mignani R et al. (2004) Enzyme replacement therapy with agalsidase beta in kidney transplant patients with Fabry disease: a pilot study. Kidney Int 65: 1381–1385
Schiffmann R (2007) Enzyme replacement in Fabry disease: the essence is in the kidney. Ann Intern Med 146: 142–144
Fogo A et al. (2006) Developing a chronicity index for kidney involvement in Fabry disease; report of the International Fabry kidney biopsy working group [abstract]. J Am Soc Nephrol 17: 626A
Oliveira JP (2007) Staging of Fabry disease using renal biopsies. Clin Ther 29: S15–S16
Acknowledgements
This Review is based on a discussion of the available evidence by an international panel of experts that met in June 2007 at the 7th International Fabry Nephropathy Roundtable, Barcelona, which was sponsored by Genzyme. The authors appreciate the assistance of Excerpta Medica in preparing the transcript of the roundtable. A Ortiz is supported by ISCIII-RETIC REDinREN/RD06/0016, Programa Intensificación Actividad Investigadora (ISCIII/Agencia Laín-Entralgo/CM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
A Ortiz is a member of the European Advisory Board of the Genzyme-sponsored Fabry Registry, acts as a consultant for Genzyme on Fabry disease, and has received honoraria from Genzyme for lectures. JP Oliveira is a member of the European Advisory Board of the Fabry Registry, and has received payment for lecture fees and support for research activities from Genzyme. C Wanner is a member of the European Advisory Board of the Fabry Registry, and has received lecture fees and support for research activities from Genzyme. BM Brenner is a Fabry disease consultant for, and has received honoraria for lectures from, Genzyme. S Waldek is a member of the European Advisory Board of the Fabry Registry, and has received lecture fees and support for research activities from Genzyme. DG Warnock is a member of the North American Advisory Board of the Fabry Registry, a consultant for Genzyme Corporation on Fabry disease, and has received honoraria for lectures and research grants from Genzyme.
Supplementary information
Supplementary Table 1
Clinical practice recommendations and guidelines for the management of Fabry nephropathy in adults. (DOC 59 kb)
Rights and permissions
About this article
Cite this article
Ortiz, A., Oliveira, J., Wanner, C. et al. Recommendations and guidelines for the diagnosis and treatment of Fabry nephropathy in adults. Nat Rev Nephrol 4, 327–336 (2008). https://doi.org/10.1038/ncpneph0806
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncpneph0806
This article is cited by
-
Impact of kidney biopsy on deciding when to initiate enzyme replacement therapy in children with Fabry disease
Pediatric Nephrology (2024)
-
Morbus Fabry
Der Kardiologe (2021)
-
Ultrastructural deposits appearing as “zebra bodies” in renal biopsy: Fabry disease?– comparative case reports
BMC Nephrology (2017)
-
Urinary mulberry cells and mulberry bodies are useful tool to detect late-onset Fabry disease
CEN Case Reports (2017)
-
Review and evaluation of the methodological quality of the existing guidelines and recommendations for inherited neurometabolic disorders
Orphanet Journal of Rare Diseases (2015)