Abstract
Nutritional and other environmental cues during development can permanently alter the structure, homeostatic systems, and functions of the body. This phenomenon has been referred to as 'programming'. Epidemiological and animal studies show that programmed effects operate within the normal range of growth and development, and influence the risk of chronic disease in adult life. We review the evidence that these effects include reduced nephron number and compensatory adaptations, which might lead to hypertension, and perhaps accelerate the decline in renal function that accompanies aging. These processes might be exacerbated by programmed changes in vascular structure and function, and alterations in endocrine and metabolic homeostasis. Programmed effects might be initiated as early as the periconceptual phase of development, and could involve epigenetic changes in gene expression or altered stem cell allocation. Better understanding of these processes could lead to the development of novel diagnostic and preventive measures, and to early detection of at-risk individuals. By monitoring blood pressure, weight, and renal function in children, it might be possible to reduce the risk of cardiovascular and renal disease in later life.
Key Points
-
According to the 'fetal origins hypothesis', different forms of cardiovascular disease and type 2 diabetes originate from 'developmental plasticity', in response to undernutrition during fetal life and infancy
-
Mechanisms through which the path of development initiates hypertension include allocation of stem cells and alteration of gene expression in the embryo; changes in renal growth; and alterations in homeostatic set-points that control blood pressure
-
These changes can make affected systems more vulnerable to disruptive influences in postnatal life including obesity, environmental stress, oxidative stress, and high salt intake
-
By monitoring blood pressure, weight, diet, and renal function in children, it might be possible to reduce the risk of cardiovascular and renal disease in later life
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
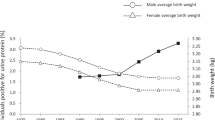
Barker DJP et al. (1989) Weight in infancy and death from ischaemic heart disease. Lancet 2: 577–580
Barker DJP (1995) Fetal origins of coronary heart disease. BMJ 311: 171–174
West-Eberhard MJ (2003) Developmental Plasticity and Evolution. Oxford: Oxford University Press
Hinchliffe SA et al. (1992) The effect of intrauterine growth retardation on the development of renal nephrons. Br J Obstet Gynaecol 99: 296–301
Mackenzie HS et al. (1995) Fewer nephrons at birth; a missing link in the etiology of essential hypertension? Am J Kidney Dis 26: 91–98
Widdowson EM and McCance RA (1963) The effect of finite periods of undernutrition at different ages on the composition and subsequent development of the rat. Proc R Soc Lond B Biol Sci 158: 329–342
Kwong WY et al. (2000) Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development 127: 4195–4202
Khan I et al. (2004) Predictive adaptive responses to maternal high-fat diet prevent endothelial dysfunction but not hypertension in adult rat offspring. Circulation 110: 1097–1102
Bateson P (2001) Fetal experience and good adult design. Int J Epidemiol 5: 928–934
Bertram CE and Hanson MA (2001) Animal models and programming of the metabolic syndrome. Br Med Bull 60: 103–121
Bateson P et al. (2004) Developmental plasticity and human health. Nature 430: 419–421
Gluckman PD et al. (2004) Living with the past: evolution, development, and patterns of disease. Science 305: 1733–1736
Barker DJ et al. (2002) Growth and living conditions in childhood and hypertension in adult life: a longitudinal study. J Hypertens 20: 1951–1956
Barker DJP et al. (2002) Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 31: 1235–1239
McCance RA (1962) Food, growth and time. Lancet 2: 621–626
Harding JE (2001) The nutritional basis of the fetal origins of adult disease. Int J Epidemiol 30: 15–23
Langley-Evans SC et al. (1999) Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci 64: 965–974
Ozaki T et al. (2001) Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. J Physiol 530: 141–152
Woods LL et al. (2004) Programming of adult blood pressure by maternal protein restriction: role of nephrogenesis. Kidney Int 65: 1339–1348
Hanson MA et al. (2005) Developmental processes and the induction of cardiovascular function: conceptual aspects. J Physiol 565: 27–34
Lane N et al. (2003) Resistance of IAPs to methylation reprogramming may provide a mechanism for epigenetic inheritance in the mouse. Genesis 35: 88–93
Lillycrop K et al. (2005) Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr 135: 1382–1386
Pham TD et al. (2003) Uteroplacental insufficiency increases apoptosis and alters p53 gene methylation in the full-term IUGR rat kidney. Am J Physiol Regul Integr Comp Physiol 285: R962–R970
Watkins A et al. (2006) The influence of mouse Ped gene expression on postnatal development. J Physiol 15: 211–220
Luycrx VA (2005) Low birth weight, nephron number, and kidney disease. Kidney Int 97 (Suppl): S68–S77
Gubhaju L et al. (2005) The baboon as a good model for studies of human kidney development. Ped Res 58: 505–509
Hughson M et al. (2003) Glomerular number and size in autopsy kidneys: the relationship to birthweight. Kidney Int 63: 2113–2122
Brenner BM and Chertow GM (1993) Congenital oligonephropathy: an inborn cause of adult hypertension and progressive renal injury? Curr Opin Nephrol Hypertens 2: 691–695
Woods LL et al. (2001) Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res 49: 460–467
Vehaskari VM et al. (2001) Prenatal programming of adult hypertension in the rat. Kidney Int 59: 238–245
Lelievre-Pegorier M et al. (1998) Mild vitamin A deficiency leads to inborn nephron deficit in the rat. Kidney Int 54: 1455–1462
Merlet-Benichou C et al. (1994) Intrauterine growth retardation leads to a permanent nephron deficit in the rat. Pediatr Nephrol 8: 175–180
Spencer J et al. (2001) Low birth weight and reduced renal volume in Aboriginal children. Am J Kidney Dis 37: 915–920
Silver LE et al. (2003) Intrauterine growth restriction is accompanied by decreased renal volume in the human fetus. Am J Obstet Gynecol 188: 1320–1325
Manalich R et al. (2000) Relationship between weight at birth and the number and size of renal glomeruli in humans: a histomorphometric study. Kidney Int 58: 770–773
Konje JC et al. (1997) A cross sectional study of changes in fetal renal size with gestation in appropriate- and small-for-gestational age fetuses. Ultrasound Obstet Gynaecol 9: 22–26
Praga M et al. (2000) Influence of obesity on the appearance of proteinuria and renal insuffiency after unilateral nephrectomy. Kidney Int 58: 2111–2118
Huxley R et al. (2002) Unravelling the fetal origins hypothesis. Lancet 360: 2074–2075
Keller G et al. (2003) Nephron number in patients with primary hypertension. N Engl J Med 348: 101–108
Ingelfinger JR (2003) Is microanatomy destiny? N Engl J Med 348: 99–100
Gossmann J et al. (2005) Long-term consequences of live kidney donation follow-up in 93% of living kidney donors in a single transplant center. Am J Transplant 5: 2417–2424
Smith S et al. (1985) Long-term effect of uninephrectomy on serum creatinine concentration and arterial blood pressure. Am J Kidney Disease 6: 143–148
Hoy WE et al. (1999) A new dimension to the Barker hypothesis: low birthweight and susceptibility to renal disease. Kidney Int 56: 1072–1077
Sanders MW et al. (2005) High sodium intake increases blood pressure and alters renal function in intrauterine growth-retarded rats. Hypertension 46: 71–75
Regina S et al. (2001) Intrauterine food restriction as a determinant of nephrosclerosis. Am J Kidney Dis 37: 467–476
Lackland DT et al. (2000) Low birth weights contribute to high rates of early-onset chronic kidney failure in the Southeastern United States. Arch Intern Med 160: 1472–1476
Mathews SG (2002) Early programming of the hypothalmo pituitary-adrenal axis. Trends Endocrinol Metab 13: 363–408
Phillips DI and Jones A (2006) Fetal programming of autonomic and HPA function: do people who were small babies have enhanced stress responses? J Physiol 572: 45–50
Clark PM et al. (1996) Size at birth and adrenocortical function in childhood. Clin Endocrinol 45: 721–726
Economides DL et al. (1988) Plasma cortisol and adrenocorticotrophin in appropriate and small gestational age fetuses. Fetal Ther 3: 158–164
Spassov L et al. (1994) Heart rate and heart rate variability during sleep in small-for-gestational age newborns. Pediatr Res 35: 500–505
Phillips DIW et al. (1997) Association between low birthweight and high resting pulse in adult life: is the sympathetic nervous system involved in programming the insulin resistance syndrome? Diabetic Med 14: 673–677
Alexander B et al. (2005) Renal denervation abolishes hypertension in low-birth-weight offspring from pregnant rats with reduced uterine perfusion. Hypertension 45: 754–758
Pladys P et al. (2004) Role of brain and peripheral angiotensin II in hypertension and altered arterial baroreflex programmed during fetal life in rat. Pediatr Res 55: 1042–1049
Matthews KA et al. (2004) Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation 110: 74–78
Allen MT et al. (1997) Cardiovascular reactivity to stress and left ventricular mass in youth. Hypertension 30: 782–787
Everson SA et al. (1997) Interaction of workplace demands and cardiovascular reactivity in progression of carotid atherosclerosis: population based study. BMJ 314: 553–558
Leeson CP et al. (1997) Flow-mediated dilation in 9- to 11-year-old children: the influence of intrauterine and childhood factors. Circulation 96: 2233–2238
Martin H et al. (2000) Impaired endothelial function and increased carotid stiffness in 9-year-old children with low birthweight. Circulation 102: 2739–2744
Brawley L et al. (2003) Dietary protein restriction in pregnancy induces hypertension and vascular defects in rat male offspring. Pediatr Res 54: 83–90
Palinski W et al. (2002) The fetal origins of atherosclerosis: maternal hypercholesterolemia, and cholesterol-lowering or antioxidant treatment during pregnancy influence in utero programming and postnatal susceptibility to atherogenesis. FASEB J 16: 1348–1360
Ahokas RA et al. (1983) Cardiac output and uteroplacental blood flow in diet-restricted and diet-repleted rats. Am J Obstet Gynaecol 146: 6–13
Itoh S et al. (2002) Vasodilation to vascular endothelial growth factor in the uterine artery of the pregnant rat is blunted by low dietary protein intake. Pediatr Res 51: 485–491
Torrens C et al. (2002) Maternal protein restriction in the rat impairs resistance artery but not conduit artery function in pregnant offspring. J Physiol 547: 77–84
Chapman N et al. (1997) Retinal vascular network architecture in low-birth-weight men. J Hypertens 15: 14449–14453
Kistner A et al. (2002) Low gestational age associated with abnormal retinal vascularization and increased blood pressure in adult women. Pediatr Res 51: 675–680
Pladys P et al. (2005) Microvascular rarefaction and decreased angiogenesis in rats with fetal programming of hypertension associated with exposure to a low-protein diet in utero. Am J Physiol 298: R1580–R1588
Hales CN and Barker DJ (1992) Type 2 (non-insulin dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetalogia 35: 595–601
Phillips DIW et al. (1996) Insulin resistance as a programmed response to fetal undernutrition. Diabetologia 39: 1119–1122
Vehaskari VM et al. (2004) Kidney angiotensin and angiotensin receptor expression in prenatally programmed hypertension. Am J Physiol Renal Physiol 287: F262–F267
Sahajpal V and Ashton N (2005) Increased glomerular angiotensin II binding in rats exposed to a maternal low protein diet in utero. J Physiol 15: 193–201
Sahajpal V and Ashton N (2003) Renal function and angiotensin AT1 receptor expression in young rats following intrauterine exposure to a maternal low-protein diet. Clin Sci (Lond) 104: 607–614
McMullen S et al. (2004) Prenatal programming of angiotensin II type 2 receptor expression in the rat. Brit J Nutr 91: 133–140
Tufro-McReddie A et al. (1995) Angiotensin II regulates nephrogenesis and renal vascular development. Am J Physiol 269: F110–F115
Gribouval O et al. (2005) Mutations in genes in the renin-angiotensin system are associated with autosomal recessive renal tubular dysgenesis. Nat Genet 37: 964–968
Reviere G et al. (2005) Angiotensin-converting enzyme 2 (ACE2) and ACE activities display tissue-specific sensitivity to undernutrition-programmed hypertension in the adult rat. Hypertension 46: 1169–1174
Langley-Evans SC et al. (1999) Intrauterine programming of hypertension: the role of the renin-angiotensin system. Biochem Soc Trans 27: 88–93
Thomson SC et al. (2004) Kidney function in early diabetes: the tubular hypothesis of glomerular filtration. Am J Physiol Renal Physiol 286: F8–F15
Yliharsila H et al. (2003) Self-perpetuating effects of birth size on blood pressure levels in elderly people. Hypertension 41: 441–450
Koupil I et al. (2005) Birthweight hypertension and 'white coat' hypertension: size at birth in relation to office and 24-hour ambulatory blood pressure. J Human Hypertens 19: 635–642
Hardy R et al. (2003) Birthweight, childhood social class, and change in adult blood pressure in the 1946 British birth cohort. Lancet 362: 1178–1183
Lackland DT et al. (2002) Associations between birthweight and antihypertensive medication in black and white medicaid recipients. Hypertension 39: 179–183
Eriksson JG et al. Fetal, infant and childhood growth and hypertension in later life: longitudinal study. BMJ, in press
Barker DJ et al. The maternal and social origins of hypertension. BMJ, in press
Acknowledgements
DJP Barker and MA Hanson received support from the British Heart Foundation. SP Bagby is supported by NIH National Institute of Child Health & Human Development grant RO1HD042570.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Barker, D., Bagby, S. & Hanson, M. Mechanisms of Disease: in utero programming in the pathogenesis of hypertension. Nat Rev Nephrol 2, 700–707 (2006). https://doi.org/10.1038/ncpneph0344
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpneph0344
This article is cited by
-
A Reduction in Antenatal Steroid Dose Was Associated with Reduced Cardiac Dysfunction in a Sheep Model of Pregnancy
Reproductive Sciences (2023)
-
FoxO1 Regulates Neuropeptide Y and Pro-opiomelanocortin in the Hypothalamus of Rat Offspring Small for Gestational Age
Reproductive Sciences (2022)
-
Progenitor translatome changes coordinated by Tsc1 increase perception of Wnt signals to end nephrogenesis
Nature Communications (2021)
-
Modulation by antenatal therapies of cardiovascular and renal programming in male and female offspring of preeclamptic rats
Naunyn-Schmiedeberg's Archives of Pharmacology (2021)
-
Epigenome-wide association study of kidney function identifies trans-ethnic and ethnic-specific loci
Genome Medicine (2021)