Abstract
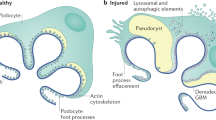
This Review summarizes recent research on the podocyte slit diaphragm. A growing number of molecules that function at the slit diaphragm have been identified in patients with inherited and sporadic nephrotic syndromes. Genetic deletion of nearly all of these molecules results in proteinuria and effacement of foot processes. Nephrin, Neph1 and podocin seem to form a multifunctional receptor complex at the slit diaphragm. Most of the other components of the slit diaphragm interact directly with this complex, in many cases coupling slit diaphragm components to the podocyte's actin cytoskeleton. These molecular findings are being applied to patients with glomerular disease. Over the next decade, these data might help to improve disease classification and prediction of which patients will respond to immunosuppressive treatment.
Key Points
-
Mutations of molecules that function at the podocyte slit diaphragm of the glomerular filter have recently been identified in patients with inherited and sporadic nephrotic syndromes
-
Experimental deletion of many slit diaphragm molecules induces the proteinuria and effacement of podocyte foot processes that are characteristic of all glomerular diseases
-
Data indicate that three molecules susceptible to mutation—nephrin, Neph1 and podocin—form a receptor complex at the slit diaphragm that interacts with the podocyte actin cytoskeleton
-
Determining the slit diaphragm genetic profile of patients with glomerular diseases should improve disease classification and tailoring of treatment
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
US Renal Data System (2004) USRDS 2004 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
D'Amico G and Bazzi C (2003) Pathophysiology of proteinuria. Kidney Int 63: 809–825.
Tryggvason K and Wartiovaara J (2005) How does the kidney filter plasma? Physiology 20: 96–101
Haraldsson B and Sorensson J (2004) Why do we not all have proteinuria? An update of our current understanding of the glomerular barrier. News Physiol Sci 19: 7–10
Deen WM et al. (2001) Structural determinants of glomerular permeability. Am J Physiol Renal Physiol 281: F579–596
Madsen KM and Tisher CC (2004) Brenner and Rector's The Kidney. In The Nephron edn 7 (Ed. Brenner BM) Philadelphia: Elsevier.
Karnovsky MJ and Ainsworth SK (1972) The structural basis of glomerular filtration. Adv Nephrol Necker Hosp 2: 35–60
Kanwar YS et al. (1991) Current status of the structural and functional basis of glomerular filtration and proteinuria. Semin Nephrol 11: 390–413
Caulfield JP and Farquhar MG (1974) The permeability of glomerular capillaries to graded dextrans: identification of the basement membrane as the primary filtration barrier. J Cell Biol 63: 883–903
Kestila M et al. (1998) Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582
Ruotsalainen V et al. (1999) Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci USA 96: 7962–7967
Holthofer H et al. (1999) Nephrin localizes at the podocyte filtration slit area and is characteristically spliced in the human kidney. Am J Pathol 155: 1681–1687
Holzman LB et al. (1999) Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int 56: 1481–1491
Barletta GM et al. (2003) Nephrin and Neph1 co-localize at the podocyte foot process intercellular junction and form cis hetero-oligomers. J Biol Chem 278: 19266–19271
Gerke P et al. (2003) Homodimerization and heterodimerization of the glomerular podocyte proteins nephrin and NEPH1. J Am Soc Nephrol 14: 918–926
Tryggvason K et al. (1999) Discovery of the congenital nephrotic syndrome gene discloses the structure of the mysterious molecular sieve of the kidney. Int J Dev Biol 43: 445–451
Wartiovaara J et al. (2004) Nephrin strands contribute to a porous slit diaphragm scaffold as revealed by electron tomography. J Clin Invest 114: 1475–1483
Rossi M et al. (2003) Heparan sulfate chains of perlecan are indispensable in the lens capsule but not in the kidney. EMBO J 22: 236–245
Smithies O (2003) Why the kidney glomerulus does not clog: a gel permeation/diffusion hypothesis of renal function. Proc Natl Acad Sci USA 100: 4108–4113
Deen WM (2004) What determines glomerular capillary permeability? J Clin Invest 114: 1412–1414
Donoviel DB et al. (2001) Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol Cell Biol 21: 4829–4836
Sellin L et al. (2003) NEPH1 defines a novel family of podocin interacting proteins. FASEB J 17: 115–117
Boute N et al. (2000) NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354
Roselli S et al. (2002) Podocin localizes in the kidney to the slit diaphragm area. Am J Pathol 160: 131–139
Salzer U and Prohaska R (2001) Stomatin, flotillin-1, and flotillin-2 are major integral proteins of erythrocyte lipid rafts. Blood 97: 1141–1143
Harder T (2004) Lipid raft domains and protein networks in T-cell receptor signal transduction. Curr Opin Immunol 16: 353–359
Simons K and Toomre D (2000) Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1: 31–39.
Simons M et al. (2001) Involvement of lipid rafts in nephrin phosphorylation and organization of the glomerular slit diaphragm. Am J Pathol 159: 1069–1077
Schwarz K et al. (2001) Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest 108: 1621–1629
Huber TB et al. (2003) Molecular basis of the functional podocin–nephrin complex: mutations in the NPHS2 gene disrupt nephrin targeting to lipid raft microdomains. Hum Mol Genet 12: 3397–3405
Shen K and Bargmann CI (2003) The immunoglobulin superfamily protein SYG-1 determines the location of specific synapses in C. elegans. Cell 112: 619–630
Shen K et al. (2004) Synaptic specificity is generated by the synaptic guidepost protein SYG-2 and its receptor, SYG-1. Cell 116: 869–881
Bao S and Cagan R (2005) Preferential adhesion mediated by Hibris and Roughest regulates morphogenesis and patterning in the Drosophila eye. Dev Cell 8: 925–935
Grzeschik NA and Knust E (2005) IrreC/rst-mediated cell sorting during Drosophila pupal eye development depends on proper localisation of DE-cadherin. Development 132: 2035–2045
Zhang S et al. (2004) MEC-2 is recruited to the putative mechanosensory complex in C. elegans touch receptor neurons through its stomatin-like domain. Curr Biol 14: 1888–1896
Liu G et al. (2003) Neph1 and nephrin interaction in the slit diaphragm is an important determinant of glomerular permeability. J Clin Invest 112: 209–221
Gumbiner BM (2005) Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol 6: 622–634
Schnabel E et al. (1990) The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol 111: 1255–1263
Huber TB et al. (2003) The carboxyl terminus of Neph family members binds to the PDZ domain protein zonula occludens-1. J Biol Chem 278: 13417–13421
Lehtonen S et al. (2005) Cell junction-associated proteins IQGAP1, MAGI-2, CASK, spectrins, and alpha-actinin are components of the nephrin multiprotein complex. Proc Natl Acad Sci USA 102: 9814–9819
Liu XL et al. (2005) Characterization of the interactions of the nephrin intracellular domain. FEBS J 272: 228–243
Noritake J et al. (2005) IQGAP1: a key regulator of adhesion and migration. J Cell Sci 118: 2085–2092
Subauste MC et al. (2005) Vinculin controls PTEN protein level by maintaining the interaction of the adherens junction protein beta-catenin with the scaffolding protein MAGI-2. J Biol Chem 280: 5676–5681
Lee S et al. (2002) A novel and conserved protein–protein interaction domain of mammalian Lin-2/CASK binds and recruits SAP97 to the lateral surface of epithelia. Mol Cell Biol 22: 1778–1791
Li S et al. (2000) Gastric hyperplasia in mice lacking the putative Cdc42 effector IQGAP1. Mol Cell Biol 20: 697–701
Inoue T et al. (2001) FAT is a component of glomerular slit diaphragms. Kidney Int 59: 1003–1012
Tanoue T and Takeichi M (2005) New insights into Fat cadherins. J Cell Sci 118: 2347–2353
Ciani L et al. (2003) Mice lacking the giant protocadherin mFAT1 exhibit renal slit junction abnormalities and a partially penetrant cyclopia and anophthalmia phenotype. Mol Cell Biol 23: 3575–3582
Moeller MJ et al. (2004) Protocadherin FAT1 binds Ena/VASP proteins and is necessary for actin dynamics and cell polarization. EMBO J 23: 3769–3779
Tanoue T and Takeichi M (2004) Mammalian Fat1 cadherin regulates actin dynamics and cell-cell contact. J Cell Biol 165: 517–528.
Winn MP et al. (2005) A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308: 1801–1804
Reiser J et al. (2005) TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet 37: 739–744
Rana K et al. (2003) Clinical, histopathologic, and genetic studies in nine families with focal segmental glomerulosclerosis. Am J Kidney Dis 41: 1170–1178
Nilius B and Voets T (2005) TRP channels: a TR(I)P through a world of multifunctional cation channels. Pflugers Arch 451: 1–10
Zagranichnaya TK et al. (2005) Endogenous TRPC1, TRPC3, and TRPC7 proteins combine to form native store-operated channels in HEK-293 cells. J Biol Chem 280: 29559–29569
Hisatsune C et al. (2004) Regulation of TRPC6 channel activity by tyrosine phosphorylation. J Biol Chem 279: 18887–18894
Lahdenpera J et al. (2003) Clustering-induced tyrosine phosphorylation of nephrin by Src family kinases. Kidney Int 64: 404–413
Verma R et al. (2003) Fyn binds to and phosphorylates the kidney slit diaphragm component nephrin. J Biol Chem 278: 20716–20723
Li H et al. (2004) SRC-family kinase Fyn phosphorylates the cytoplasmic domain of nephrin and modulates its interaction with podocin. J Am Soc Nephrol 15: 3006–3015
Sechi AS and Wehland J (2004) Interplay between TCR signalling and actin cytoskeleton dynamics. Trends Immunol 25: 257–265
Huber TB et al. (2003) Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol Cell Biol 23: 4917–4928
Cantley LC (2002) The phosphoinositide 3-kinase pathway. Science 296: 1655–1657
Downward J (1998) Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol 10: 262–267
Kim YH et al. (2001) Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int 60: 957–968
Dustin ML et al. (1998) A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell 94: 667–677
Shih NY et al. (1999) Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 286: 312–315
Kim JM et al. (2003) CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science 300: 1298–1300
Kobayashi S et al. (2004) The c-Cbl/CD2AP complex regulates VEGF-induced endocytosis and degradation of Flt-1 (VEGFR-1). FASEB J 18: 929–931
Kowanetz K et al. (2003) Identification of a novel proline-arginine motif involved in CIN85-dependent clustering of Cbl and down-regulation of epidermal growth factor receptors. J Biol Chem 278: 39735–39746
Cormont M et al. (2003) CD2AP/CMS regulates endosome morphology and traffic to the degradative pathway through its interaction with Rab4 and c-Cbl. Traffic 4: 97–112
Welsch T et al. (2005) Association of CD2AP with dynamic actin on vesicles in podocytes. Am J Physiol Renal Physiol 289: F1134–F1143
Tsukaguchi H et al. (2002) NPHS2 mutations in late-onset focal segmental glomerulosclerosis: R229Q is a common disease-associated allele. J Clin Invest 110: 1659–1666
Frishberg Y et al. (2002) Mutations in NPHS2 encoding podocin are a prevalent cause of steroid-resistant nephrotic syndrome among Israeli-Arab children. J Am Soc Nephrol 13: 400–405
Weber S et al. (2004) NPHS2 mutation analysis shows genetic heterogeneity of steroid-resistant nephrotic syndrome and low post-transplant recurrence. Kidney Int 66: 571–579
Grunkemeyer JA et al. (2005) CD2-associated protein (CD2AP) expression in podocytes rescues lethality of CD2AP deficiency. J Biol Chem 280: 29677–29681
Ruf RG et al. (2004) Patients with mutations in NPHS2 (podocin) do not respond to standard steroid treatment of nephrotic syndrome. J Am Soc Nephrol 15: 722–732
Ghiggeri GM et al. (2004) Cyclosporine in patients with steroid-resistant nephrotic syndrome: an open-label, nonrandomized, retrospective study. Clin Ther 26: 1411–1418
Vincenti F and Ghiggeri GM (2005) New insights into the pathogenesis and the therapy of recurrent focal glomerulosclerosis. Am J Transplant 5: 1179–1185
Henger A et al. (2004) Gene expression analysis of human renal biopsies: recent developments towards molecular diagnosis of kidney disease. Curr Opin Nephrol Hypertens 13: 313–318
Schmid H et al. (2003) Gene expression profiles of podocyte-associated molecules as diagnostic markers in acquired proteinuric diseases. J Am Soc Nephrol 14: 2958–2966
Petermann AT et al. (2003) Podocytes that detach in experimental membranous nephropathy are viable. Kidney Int 64: 1222–1231
Szeto CC et al. (2005) Messenger RNA expression of glomerular podocyte markers in the urinary sediment of acquired proteinuric diseases. Clin Chim Acta 361: 182–190
Hoorn EJ et al. (2005) Prospects for urinary proteomics: exosomes as a source of urinary biomarkers. Nephrology (Carlton) 10: 283–290
Ahola H et al. (2003) A novel protein, densin, expressed by glomerular podocytes. J Am Soc Nephrol 14: 1731–1737
Cohen CD et al.: Comparative promoter analysis allows de novo identification of specialized cell junction associated proteins. Proc Natl Acad Sci USA, in press
Kaplan JM et al. (2000) Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 24: 251–256
Yao J et al. (2004) Alpha-actinin-4-mediated FSGS: an inherited kidney disease caused by an aggregated and rapidly degraded cytoskeletal protein. PLoS Biol 2: e167.
Ichimura K et al. (2003) Actin filament organization of foot processes in rat podocytes. J Histochem Cytochem 51: 1589–1600
Reiser J et al. (2000) The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol 11: 1–8
Acknowledgements
We thank other researchers for their forbearance, as there were many interesting observations that could not be included herein because of limited space.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Johnstone, D., Holzman, L. Clinical impact of research on the podocyte slit diaphragm. Nat Rev Nephrol 2, 271–282 (2006). https://doi.org/10.1038/ncpneph0180
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpneph0180
This article is cited by
-
Porcine models for studying complications and organ crosstalk in diabetes mellitus
Cell and Tissue Research (2020)
-
Podocyte injury in diabetic nephropathy: implications of angiotensin II – dependent activation of TRPC channels
Scientific Reports (2015)
-
Crk1/2 and CrkL form a hetero-oligomer and functionally complement each other during podocyte morphogenesis
Kidney International (2014)
-
The role of the podocyte in albumin filtration
Nature Reviews Nephrology (2013)
-
Myo1c is an unconventional myosin required for zebrafish glomerular development
Kidney International (2013)