Abstract
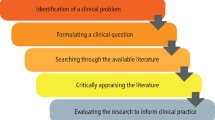
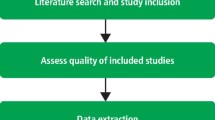
Critical appraisal is a systematic process used to identify the strengths and weaknesses of a research article in order to assess the usefulness and validity of research findings. The most important components of a critical appraisal are an evaluation of the appropriateness of the study design for the research question and a careful assessment of the key methodological features of this design. Other factors that also should be considered include the suitability of the statistical methods used and their subsequent interpretation, potential conflicts of interest and the relevance of the research to one's own practice. This Review presents a 10-step guide to critical appraisal that aims to assist clinicians to identify the most relevant high-quality studies available to guide their clinical practice.
Key Points
-
Critical appraisal is a systematic process used to identify the strengths and weaknesses of a research article
-
Critical appraisal provides a basis for decisions on whether to use the results of a study in clinical practice
-
Different study designs are prone to various sources of systematic bias
-
Design-specific, critical-appraisal checklists are useful tools to help assess study quality
-
Assessments of other factors, including the importance of the research question, the appropriateness of statistical analysis, the legitimacy of conclusions and potential conflicts of interest are an important part of the critical appraisal process
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Druss BG and Marcus SC (2005) Growth and decentralisation of the medical literature: implications for evidence-based medicine. J Med Libr Assoc 93: 499–501
Glasziou PP (2008) Information overload: what's behind it, what's beyond it? Med J Aust 189: 84–85
Last JE (Ed.; 2001) A Dictionary of Epidemiology (4th Edn). New York: Oxford University Press
Sackett DL et al. (2000). Evidence-based Medicine. How to Practice and Teach EBM. London: Churchill Livingstone
Guyatt G and Rennie D (Eds; 2002). Users' Guides to the Medical Literature: a Manual for Evidence-based Clinical Practice. Chicago: American Medical Association
Greenhalgh T (2000) How to Read a Paper: the Basics of Evidence-based Medicine. London: Blackwell Medicine Books
MacAuley D (1994) READER: an acronym to aid critical reading by general practitioners. Br J Gen Pract 44: 83–85
Hill A and Spittlehouse C (2001) What is critical appraisal. Evidence-based Medicine 3: 1–8 [http://www.evidence-based-medicine.co.uk] (accessed 25 November 2008)
Public Health Resource Unit (2008) Critical Appraisal Skills Programme (CASP). [http://www.phru.nhs.uk/Pages/PHD/CASP.htm] (accessed 8 August 2008)
National Health and Medical Research Council (2000) How to Review the Evidence: Systematic Identification and Review of the Scientific Literature. Canberra: NHMRC
Elwood JM (1998) Critical Appraisal of Epidemiological Studies and Clinical Trials (2nd Edn). Oxford: Oxford University Press
Agency for Healthcare Research and Quality (2002) Systems to rate the strength of scientific evidence? Evidence Report/Technology Assessment No 47, Publication No 02-E019 Rockville: Agency for Healthcare Research and Quality
Crombie IK (1996) The Pocket Guide to Critical Appraisal: a Handbook for Health Care Professionals. London: Blackwell Medicine Publishing Group
Heller RF et al. (2008) Critical appraisal for public health: a new checklist. Public Health 122: 92–98
MacAuley D et al. (1998) Randomised controlled trial of the READER method of critical appraisal in general practice. BMJ 316: 1134–37
Parkes J et al. Teaching critical appraisal skills in health care settings (Review). Cochrane Database of Systematic Reviews 2005, Issue 3. Art. No.: cd001270. 10.1002/14651858.cd001270
Mays N and Pope C (2000) Assessing quality in qualitative research. BMJ 320: 50–52
Hawking SW (2003) On the Shoulders of Giants: the Great Works of Physics and Astronomy. Philadelphia, PN: Penguin
National Health and Medical Research Council (1999) A Guide to the Development, Implementation and Evaluation of Clinical Practice Guidelines. Canberra: National Health and Medical Research Council
US Preventive Services Taskforce (1996) Guide to clinical preventive services (2nd Edn). Baltimore, MD: Williams & Wilkins
Solomon MJ and McLeod RS (1995) Should we be performing more randomized controlled trials evaluating surgical operations? Surgery 118: 456–467
Rothman KJ (2002) Epidemiology: an Introduction. Oxford: Oxford University Press
Young JM and Solomon MJ (2003) Improving the evidence-base in surgery: sources of bias in surgical studies. ANZ J Surg 73: 504–506
Margitic SE et al. (1995) Lessons learned from a prospective meta-analysis. J Am Geriatr Soc 43: 435–439
Shea B et al. (2001) Assessing the quality of reports of systematic reviews: the QUORUM statement compared to other tools. In Systematic Reviews in Health Care: Meta-analysis in Context 2nd Edition, 122–139 (Eds Egger M. et al.) London: BMJ Books
Easterbrook PH et al. (1991) Publication bias in clinical research. Lancet 337: 867–872
Begg CB and Berlin JA (1989) Publication bias and dissemination of clinical research. J Natl Cancer Inst 81: 107–115
Moher D et al. (2000) Improving the quality of reports of meta-analyses of randomised controlled trials: the QUORUM statement. Br J Surg 87: 1448–1454
Shea BJ et al. (2007) Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Medical Research Methodology 7: 10 [10.1186/1471-2288-7-10]
Stroup DF et al. (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283: 2008–2012
Young JM and Solomon MJ (2003) Improving the evidence-base in surgery: evaluating surgical effectiveness. ANZ J Surg 73: 507–510
Schulz KF (1995) Subverting randomization in controlled trials. JAMA 274: 1456–1458
Schulz KF et al. (1995) Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 273: 408–412
Moher D et al. (2001) The CONSORT statement: revised recommendations for improving the quality of reports of parallel group randomized trials. BMC Medical Research Methodology 1: 2 [http://www.biomedcentral.com/ 1471-2288/1/2] (accessed 25 November 2008)
Rochon PA et al. (2005) Reader's guide to critical appraisal of cohort studies: 1. Role and design. BMJ 330: 895–897
Mamdani M et al. (2005) Reader's guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. BMJ 330: 960–962
Normand S et al. (2005) Reader's guide to critical appraisal of cohort studies: 3. Analytical strategies to reduce confounding. BMJ 330: 1021–1023
von Elm E et al. (2007) Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 335: 806–808
Sutton-Tyrrell K (1991) Assessing bias in case-control studies: proper selection of cases and controls. Stroke 22: 938–942
Knottnerus J (2003) Assessment of the accuracy of diagnostic tests: the cross-sectional study. J Clin Epidemiol 56: 1118–1128
Furukawa TA and Guyatt GH (2006) Sources of bias in diagnostic accuracy studies and the diagnostic process. CMAJ 174: 481–482
Bossyut PM et al. (2003)The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med 138: W1–W12
STARD statement (Standards for the Reporting of Diagnostic Accuracy Studies). [http://www.stard-statement.org/] (accessed 10 September 2008)
Raftery J (1998) Economic evaluation: an introduction. BMJ 316: 1013–1014
Palmer S et al. (1999) Economics notes: types of economic evaluation. BMJ 318: 1349
Russ S et al. (1999) Barriers to participation in randomized controlled trials: a systematic review. J Clin Epidemiol 52: 1143–1156
Tinmouth JM et al. (2004) Are claims of equivalency in digestive diseases trials supported by the evidence? Gastroentrology 126: 1700–1710
Kaul S and Diamond GA (2006) Good enough: a primer on the analysis and interpretation of noninferiority trials. Ann Intern Med 145: 62–69
Piaggio G et al. (2006) Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA 295: 1152–1160
Heritier SR et al. (2007) Inclusion of patients in clinical trial analysis: the intention to treat principle. In Interpreting and Reporting Clinical Trials: a Guide to the CONSORT Statement and the Principles of Randomized Controlled Trials, 92–98 (Eds Keech A. et al.) Strawberry Hills, NSW: Australian Medical Publishing Company
National Health and Medical Research Council (2007) National Statement on Ethical Conduct in Human Research 89–90 Canberra: NHMRC
Lo B et al. (2000) Conflict-of-interest policies for investigators in clinical trials. N Engl J Med 343: 1616–1620
Kim SYH et al. (2004) Potential research participants' views regarding researcher and institutional financial conflicts of interests. J Med Ethics 30: 73–79
Komesaroff PA and Kerridge IH (2002) Ethical issues concerning the relationships between medical practitioners and the pharmaceutical industry. Med J Aust 176: 118–121
Little M (1999) Research, ethics and conflicts of interest. J Med Ethics 25: 259–262
Lemmens T and Singer PA (1998) Bioethics for clinicians: 17. Conflict of interest in research, education and patient care. CMAJ 159: 960–965
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Young, J., Solomon, M. How to critically appraise an article. Nat Rev Gastroenterol Hepatol 6, 82–91 (2009). https://doi.org/10.1038/ncpgasthep1331
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncpgasthep1331
This article is cited by
-
Emergency physicians’ perceptions of critical appraisal skills: a qualitative study
BMC Medical Education (2022)
-
An integrative review on individual determinants of enrolment in National Health Insurance Scheme among older adults in Ghana
BMC Primary Care (2022)
-
Autopsy findings of COVID-19 in children: a systematic review and meta-analysis
Forensic Science, Medicine and Pathology (2022)
-
The use of a modified Delphi technique to develop a critical appraisal tool for clinical pharmacokinetic studies
International Journal of Clinical Pharmacy (2022)
-
Critical Appraisal: Analysis of a Prospective Comparative Study Published in IJS
Indian Journal of Surgery (2021)