Abstract
Advances in imaging techniques and in molecular diagnosis have enabled the identification in the fetus of disorders of thyroid and adrenal function that can potentially be treated in utero through the mother. In women with Graves disease, the rare instances of autoimmune fetal hyperthyroidism can generally be treated in a noninvasive way by optimizing treatment of the mother. For fetal hypothyroidism with goiter leading to hydramnios, repeated intra-amniotic injections of thyroxine have been reported to decrease the size of the fetal thyroid, but experience is limited and the risk of premature labor is raised. In women who have previously borne a child with severe congenital adrenal hyperplasia, attempts to prevent virilization of the external genitalia of further affected female fetuses involves treatment with high doses of dexamethasone from week 7 of gestation to term, which includes the crucial period of organogenesis. Only one of every eight fetuses treated will, however, benefit from this therapy, meaning that seven are unnecessarily exposed to this potentially harmful agent. In this article, we review the rationale and evidence for efficacy of these approaches, and discuss their potential adverse effects as well as the ethical problems that they raise.
Key Points
-
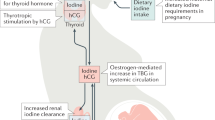
Iodine intake should be optimized in all pregnant women
-
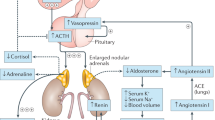
Women with present or past Graves disease should be carefully followed when they become pregnant to detect thyroid dysfunction in their fetus
-
Fetal goiters can now be revealed by second-trimester echography and, if associated with fetal hypothyroidism, might require intra-amniotic levothyroxine administration
-
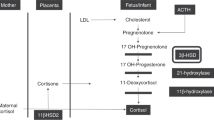
Virilization of female fetuses with congenital adrenal hyperplasia can be prevented effectively by treating the mother with dexamethasone from 5 weeks of gestation; however, this strategy might unnecessarily expose unaffected fetuses to this treatment, which could have long-term undesired effects
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Di Cosmo C et al. (2006) The sodium-iodide symporter expression in placental tissue at different gestational age: an immunohistochemical study. Clin Endocrinol (Oxf) 65: 544–548
Cao XY et al. (1994) Timing of vulnerability of the brain to iodine deficiency in endemic cretinism. N Engl J Med 331: 1739–1744
Delange F et al. (2001) Iodine deficiency in the world: where do we stand at the turn of the century. Thyroid 11: 437–447
Glinoer D (2007) The importance of iodine nutrition during pregnancy. Public Health Nutr 10: 1542–1546
Calvo RM et al. (2002) Fetal tissues are exposed to biologically relevant free thyroxine concentrations during early phases of development. J Clin Endocrinol Metab 87: 1768–1777
Vulsma T et al. (1989) Maternal-fetal transfer of thyroxine in congenital hypothyroidism due to a total organification defect of thyroid agenesis. N Engl J Med 321: 13–16
Anselmo J et al. (2004) Fetal loss associated with excess thyroid hormone exposure. JAMA 292: 691–695
Chan SY et al. (2006) Monocarboxylate transporter 8 expression in the human placenta: the effects of severe intrauterine growth restriction. J Endocrinol 189: 465–471
de Zegher F et al. (1995) The prenatal role of thyroid hormone evidenced by fetomaternal Pit-1 deficiency. J Clin Endocrinol Metab 80: 3127–3130
Haddow JE et al. (1999) Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 341: 549–555
Pop VJ et al. (1999) Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol 50: 147–148
Glinoer D and Delange F (2000) The potential repercussions of maternal, fetal, and neonatal hypothyroxinemia on the progeny. Thyroid 10: 871–887
Alexander EK et al. (2004) Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. N Engl J Med 351: 241–249
Miyata I et al. (2007) Successful intrauterine therapy for fetal goitrous hypothyroidism during late gestation. Endocr J 54: 813–817
Borgel K et al. (2005) Intrauterine therapy of goitrous hypothyroidism in a boy with a new compound heterozygous mutation (Y453D and C800R) in the thyroid peroxidase gene: a long-term follow-up. Am J Obstet Gynecol 193: 857–858
Calvo R et al. (1990) Congenital hypothyroidism, as studied in rats: crucial role of maternal thyroxine but not of 3,5,3'-triiodothyronine in the protection of the fetal brain. J Clin Invest 86: 889–899
Dubuis JM et al. (1996) Outcome of severe congenital hypothyroidism: closing the developmental gap with early high dose levothyroxine treatment. J Clin Endocrinol Metab 81: 222–227
Simoneau-Roy J et al. (2004) Cognition and behavior at school entry in children with congenital hypothyroidism treated early with high-dose levothyroxine. J Pediatr 144: 747–752
Perelman AH et al. (1990) Intrauterine diagnosis and treatment of fetal goitrous hypothyroidism. J Clin Endocrinol Metab 71: 618–621
Davidson KM et al. (1991) Successful in utero treatment of fetal goiter and hypothyroidism. N Engl J Med 324: 543–546
Polak M et al. (2004) Fetal and neonatal thyroid function in relation to maternal Graves' disease. Best Pract Res Clin Endocrinol Metab 18: 289–302
Zakarija M and McKenzie JM (1983) Pregnancy-associated changes in the thyroid-stimulating antibody of Graves' disease and the relationship to neonatal hyperthyroidism. J Clin Endocrinol Metab 57: 1036–1040
Karlsson FA et al. (1984) Thyroid blocking antibodies in thyroiditis. Acta Med Scand 215: 461–466
Pacaud D et al. (1995) Outcome in three siblings with antibody-mediated transient congenital hypothyroidism. J Pediatr 127: 275–277
Kopp P et al. (1995) Brief report: congenital hyperthyroidism caused by a mutation in the thyrotropin-receptor gene. N Engl J Med 332: 150–154
Kopp P et al. (1997) Congenital hyperthyroidism caused by a solitary toxic adenoma harboring a novel somatic mutation (serine281-->isoleucine) in the extracellular domain of the thyrotropin receptor. J Clin Invest 100: 1634–1639
Duprez L et al. (1994) Germline mutations in the thyrotropin receptor gene cause non-autoimmune autosomal dominant hyperthyroidism. Nat Genet 7: 396–401
de Roux N et al. (1996) A neomutation of the thyroid-stimulating hormone receptor in a severe neonatal hyperthyroidism. J Clin Endocrinol Metab 81: 2023–2026
Luton D et al. (2005) Management of Graves' disease during pregnancy: the key role of fetal thyroid gland monitoring. J Clin Endocrinol Metab 90: 6093–6098
Ranzini AC et al. (2001) Ultrasonography of the fetal thyroid: nomograms based on biparietal diameter and gestational age. J Ultrasound Med 20: 613–617
Foulds N et al. (2004) Carbimazole embryopathy: an emerging phenotype. Am J Med Genet 132A: 130–135
Wedell A et al. (1994) Mutational spectrum of the steroid 21-hydroxylase gene in Sweden: implications for genetic diagnosis and association with disease manifestation. J Clin Endocrinol Metab 78: 1145–1152
Meyer-Bahlburg HF et al. (2008) Sexual orientation in women with classical or non-classical congenital adrenal hyperplasia as a function of degree of prenatal androgen excess. Arch Sex Behav 37: 85–99
David M and Forest MG (1984) Prenatal treatment of congenital adrenal hyperplasia resulting from 21-hydroxylase deficiency. J Pediatr 105: 799–803
New MI et al. (2001) Prenatal diagnosis for congenital adrenal hyperplasia in 532 pregnancies. J Clin Endocrinol Metab 86: 5651–5657
Speiser PW and White PC (2003) Congenital adrenal hyperplasia. N Engl J Med 349: 776–788
Ghizzoni L et al. (2007) Prenatal and early postnatal treatment of congenital adrenal hyperplasia. Endocr Dev 11: 58–69
Nimkarn S and New MI (2007) Prenatal diagnosis and treatment of congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. Nat Clin Pract Endocrinol Metab 3: 405–413
Forest MG et al. (1993) Prenatal diagnosis and treatment of 21-hydroxylase deficiency. J Steroid Biochem Mol Biol 45: 75–82
Seckl JR and Miller WL (1997) How safe is long-term prenatal glucocorticoid treatment. JAMA 277: 1077–1079
Miller WL (1999) Dexamethasone treatment of congenital adrenal hyperplasia in utero: an experimental therapy of unproven safety. J Urol 162: 537–540
Seckl JR (2008) Glucocorticoids, developmental 'programming' and the risk of affective dysfunction. Prog Brain Res 167: 17–34
Guibert J et al. (2003) Kinetics of SRY gene appearance in maternal serum: detection by real time PCR in early pregnancy after assisted reproductive technique. Hum Reprod 18: 1733–1736
Lajic S et al. (1998) Long-term somatic follow-up of prenatally treated children with congenital adrenal hyperplasia. J Clin Endocrinol Metab 83: 3872–3880
Celsi G et al. (1998) Prenatal dexamethasone causes oligonephronia, sodium retention, and higher blood pressure in the offspring. Pediatr Res 44: 317–322
Neal CR Jr et al. (2004) Effect of neonatal dexamethasone exposure on growth and neurological development in the adult rat. Am J Physiol Regul Integr Comp Physiol 287: R375–R385
Emgard M et al. (2007) Prenatal glucocorticoid exposure affects learning and vulnerability of cholinergic neurons. Neurobiol Aging 28: 112–121
Novy MJ and Walsh SW (1983) Dexamethasone and estradiol treatment in pregnant rhesus macaques: effects on gestational length, maternal plasma hormones, and fetal growth. Am J Obstet Gynecol 145: 920–931
Uno H et al. (1990) Brain damage induced by prenatal exposure to dexamethasone in fetal rhesus macaques. I. Hippocampus. Brain Res Dev Brain Res 53: 157–167
Uno H et al. (1994) Neurotoxicity of glucocorticoids in the primate brain. Horm Behav 28: 336–348
French NP et al. (1999) Repeated antenatal corticosteroids: size at birth and subsequent development. Am J Obstet Gynecol 180: 114–121
Yeh TF et al. (2004) Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med 350: 1304–1313
Forest MG (1997) Prenatal diagnosis, treatment, and outcome in infants with congenital adrenal hyperplasia. Current Opinion in Endocrinology and Diabetology 4: 209–217
Carlson AD et al. (1999) Congenital adrenal hyperplasia: update on prenatal diagnosis and treatment. J Steroid Biochem Mol Biol 69: 19–29
Trautman PD et al. (1995) Effects of early prenatal dexamethasone on the cognitive and behavioral development of young children: results of a pilot study. Psychoneuroendocrinology 20: 439–449
Meyer-Bahlburg HF et al. (2004) Cognitive and motor development of children with and without congenital adrenal hyperplasia after early-prenatal dexamethasone. J Clin Endocrinol Metab 89: 610–614
Hirvikoski T et al. (2007) Cognitive functions in children at risk for congenital adrenal hyperplasia treated prenatally with dexamethasone. J Clin Endocrinol Metab 92: 542–548
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Van Vliet, G., Polak, M. & Ritzén, E. Treating fetal thyroid and adrenal disorders through the mother. Nat Rev Endocrinol 4, 675–682 (2008). https://doi.org/10.1038/ncpendmet1005
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncpendmet1005
This article is cited by
-
The detrimental effects of glucocorticoids exposure during pregnancy on offspring’s cardiac functions mediated by hypermethylation of bone morphogenetic protein-4
Cell Death & Disease (2018)
-
Glucocorticoids and fetal programming part 1: outcomes
Nature Reviews Endocrinology (2014)
-
Erratum: Treating fetal thyroid and adrenal disorders through the mother
Nature Clinical Practice Endocrinology & Metabolism (2009)