Abstract
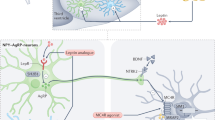
Obesity is associated with increased morbidity and mortality from cardiovascular disease, diabetes mellitus and certain cancers. The prevalence of obesity is increasing rapidly throughout the world and is now recognized as a major global public-health concern. Although the increased prevalence of obesity is undoubtedly driven by environmental factors, the evidence that inherited factors profoundly influence human fat mass is equally compelling. Twin and adoption studies indicate that up to 70% of the interindividual variance in fat mass is determined by genetic factors. Genetic strategies can, therefore, provide a useful tool with which to dissect the complex (and often heterogeneous) molecular and physiologic mechanisms involved in the regulation of body weight. In this Review, we have focused our attention on monogenic disorders, which primarily result in severe, early-onset obesity. The study of these genetic disorders has provided a framework for our understanding of the mechanisms involved in the regulation of body weight in humans and how these mechanisms are disrupted in obesity. The genes affected in these monogenic disorders all encode ligands and receptors of the highly conserved leptin–melanocortin pathway, which is critical for the regulation of food intake and body weight.
Key Points
-
Leptin regulates eating behavior, T-cell-mediated immunity and the onset of puberty
-
Mutations in the pro-opiomelanocortin gene (POMC) cause adrenocorticotropic hormone deficiency, severe obesity and hypopigmentation
-
Melanocortin receptor 4 (MC4R) deficiency is dominantly inherited with variable penetrance and expression
-
Mutation of the brain-derived neurotrophic factor gene (BDNF) and the tyrosine kinase receptor B gene (NTRK2) causes developmental delay, severe obesity, hyperactivity and impaired memory
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Barsh GS et al. (2000) Genetics of body-weight regulation. Nature 404: 644–651
Frayling TM et al. (2007) A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316: 889–894
Dina C et al. (2007) Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet 39: 724–726
Hinney A et al. (2007) Genome wide association (GWA) study for early onset extreme obesity supports the role of fat mass and obesity associated gene (FTO) variants. PLoS ONE 2: e1361
Gerken T et al. (2007) The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 318: 1469–1472
Stratigopoulos G et al. (2008) Regulation of Fto/Ftm gene expression in mice and humans. Am J Physiol Regul Integr Comp Physiol 294: R1185–R1196
Wardle J et al. (2008) Obesity-associated genetic variation in FTO is associated with diminished satiety. J Clin Endocrinol Metab [10.1210/jc.2008-0472]
Berentzen T et al. (2008) Lack of association of fatness-related FTO gene variants with energy expenditure or physical activity. J Clin Endocrinol Metab 93: 2904–2908
Loos RJ et al. (2008) Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet 40: 768–775
Zhang Y et al. (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432
Maffei M et al. (1995) Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1: 1155–1161
Ahima RS et al. (1996) Role of leptin in the neuroendocrine response to fasting. Nature 382: 250–252
Elmquist JK et al. (1998) Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 395: 535–547
Tartaglia LA (1997) The leptin receptor. J Biol Chem 272: 6093–6096
Myers MG et al. (2008) Mechanisms of leptin action and leptin resistance. Annu Rev Physiol 70: 537–556
Bates SH et al. (2003) STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421: 856–859
Schwartz MW et al. (2000) Central nervous system control of food intake. Nature 404: 661–671
Pritchard LE et al. (2002) Pro-opiomelanocortin processing in the hypothalamus: impact on melanocortin signalling and obesity. J Endocrinol 172: 411–421
Montague CT et al. (1997) Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387: 903–908
Gibson WT et al. (2004) Congenital leptin deficiency due to homozygosity for the Delta133G mutation: report of another case and evaluation of response to four years of leptin therapy. J Clin Endocrinol Metab 89: 4821–4826
Strobel A et al. (1998) A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet 18: 213–215
Ozata M et al. (1999) Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab 84: 3686–3695
Farooqi IS et al. (2007) Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med 356: 237–247
Farooqi IS et al. (2001) Partial leptin deficiency and human adiposity. Nature 414: 34–35
Lahlou N et al. (2000) Soluble leptin receptor in serum of subjects with complete resistance to leptin: relation to fat mass. Diabetes 49: 1347–1352
Farooqi IS et al. (1999) Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med 341: 879–884
Farooqi IS et al. (2007) Leptin regulates striatal regions and human eating behavior. Science 317: 1355
Clément K et al. (1998) A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 392: 398–401
Farooqi IS et al. (2002) Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest 110: 1093–1103
Licinio J et al. (2004) Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc Natl Acad Sci USA 101: 4531–4536
Heymsfield SB et al. (1999) Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 282: 1568–1575
Howard JK et al. (2004) Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med 10: 734–738
Kaszubska W et al. (2002) Protein tyrosine phosphatase 1B negatively regulates leptin signaling in a hypothalamic cell line. Mol Cell Endocrinol 195: 109–118
Klaman LD et al. (2000) Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol 20: 5479–5489
Cone RD (1999) The central melanocortin system and energy homeostasis. Trends Endocrinol Metab 10: 211–216
Coll AP et al. (2004) Proopiomelanocortin and energy balance: insights from human and murine genetics. J Clin Endocrinol Metab 89: 2557–2562
Farooqi IS et al. (2000) Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest 106: 271–279
Huszar D et al. (1997) Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88: 131–141
Butler AA et al. (2000) A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology 141: 3518–3521
Lee YS et al. (2002) A novel melanocortin 3 receptor gene (MC3R) mutation associated with severe obesity. J Clin Endocrinol Metab 87: 1423–1426
Krude H et al. (1998) Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet 19: 155–157
Krude H et al. (2003) Obesity due to proopiomelanocortin deficiency: three new cases and treatment trials with thyroid hormone and ACTH4-10. J Clin Endocrinol Metab 88: 4633–4640
Farooqi IS et al. (2006) Heterozygosity for a POMC-null mutation and increased obesity risk in humans. Diabetes 55: 2549–2553
Challis BG et al. (2002) A missense mutation disrupting a dibasic prohormone processing site in pro-opiomelanocortin (POMC) increases susceptibility to early-onset obesity through a novel molecular mechanism. Hum Mol Genet 11: 1997–2004
Echwald SM et al. (1999) Mutational analysis of the proopiomelanocortin gene in Caucasians with early onset obesity. Int J Obes Relat Metab Disord 23: 293–298
Lee YS et al. (2006) A POMC variant implicates beta-melanocyte-stimulating hormone in the control of human energy balance. Cell Metab 3: 135–140
Biebermann H et al. (2006) A role for beta-melanocyte-stimulating hormone in human body-weight regulation. Cell Metab 3: 141–146
Jackson RS et al. (2003) Small-intestinal dysfunction accompanies the complex endocrinopathy of human proprotein convertase 1 deficiency. J Clin Invest 112: 1550–1560
Jackson RS et al. (1997) Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat Genet 16: 303–306
Farooqi IS et al. (2007) Hyperphagia and early-onset obesity due to a novel homozygous missense mutation in prohormone convertase 1/3. J Clin Endocrinol Metab 92: 3369–3373
Pogozheva ID et al. (2005) Interactions of human melanocortin 4 receptor with nonpeptide and peptide agonists. Biochemistry 44: 11329–11341
Farooqi IS et al. (2003) Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med 348: 1085–1095
Alharbi KK et al. (2007) Prevalence and functionality of paucimorphic and private MC4R mutations in a large, unselected European British population, scanned by meltMADGE. Hum Mutat 28: 294–302
Vaisse C et al. (2000) Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Invest 106: 253–262
Stutzmann F et al. (2008) Prevalence of MC4R deficiency in European population and their age-dependant penetrance in multi-generational pedigrees. Diabetes [10.2337/db08-0153]
Nogueiras R et al. (2007) The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest 117: 3475–3488
Yeo GS et al. (2003) Mutations in the human melanocortin-4 receptor gene associated with severe familial obesity disrupts receptor function through multiple molecular mechanisms. Hum Mol Genet 12: 561–574
Lubrano-Berthelier C et al. (2006) Melanocortin 4 receptor mutations in a large cohort of severely obese adults: prevalence, functional classification, genotype-phenotype relationship, and lack of association with binge eating. J Clin Endocrinol Metab 91: 1811–1818
Jones KR et al. (1994) Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell 76: 989–999
Kernie SG et al. (2000) BDNF regulates eating behavior and locomotor activity in mice. EMBO J 19: 1290–1300
Xu B et al. (2003) Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci 6: 736–742
Yeo GS et al. (2004) A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat Neurosci 7: 1187–1189
Gray J et al. (2006) Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes 55: 3366–3371
Otvos L Jr et al. (2008) Development of a pharmacologically improved peptide agonist of the leptin receptor. Biochim Biophys Acta [10.1016/j.bbamcr.2008.05.007]
Hsiung HM et al. (2005) A novel and selective beta-melanocyte-stimulating hormone-derived peptide agonist for melanocortin 4 receptor potently decreased food intake and body weight gain in diet-induced obese rats. Endocrinology 146: 5257–5266
Emmerson PJ et al. (2007) Melanocortin-4 receptor agonists for the treatment of obesity. Curr Top Med Chem 7: 1121–1130
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Farooqi, I., O'Rahilly, S. Mutations in ligands and receptors of the leptin–melanocortin pathway that lead to obesity. Nat Rev Endocrinol 4, 569–577 (2008). https://doi.org/10.1038/ncpendmet0966
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncpendmet0966
This article is cited by
-
Relevance and consequence of chronic inflammation for obesity development
Molecular and Cellular Pediatrics (2023)
-
Rare genetic forms of obesity in childhood and adolescence, a comprehensive review of their molecular mechanisms and diagnostic approach
European Journal of Pediatrics (2023)
-
The promise of new anti-obesity therapies arising from knowledge of genetic obesity traits
Nature Reviews Endocrinology (2022)
-
Effects of Heterozygous Variants in the Leptin-Melanocortin Pathway on Roux-en-Y Gastric Bypass Outcomes: a 15-Year Case–Control Study
Obesity Surgery (2022)
-
Molecular genetic diagnostics of hypogonadotropic hypogonadism: from panel design towards result interpretation in clinical practice
Human Genetics (2021)