Abstract
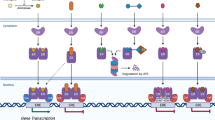
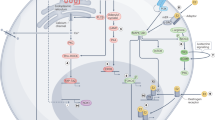
The first-generation selective estrogen receptor modulator (SERM) tamoxifen has been the mainstream hormone therapy in breast cancer. Tamoxifen benefits all stages of the disease, but its use increases the risk of uterine cancer and thromboembolic events and it can only be administered for 5 years. Aromatase inhibitors are superior to tamoxifen at advanced stages of disease and as adjuvants; however, because they increase fractures, aromatase inhibitors are unlikely to be used to prevent disease. Raloxifene, a second-generation SERM, leads, like tamoxifen, to approximately 50% fewer cases of invasive breast cancer in high risk women, with a lower incidence of thromboembolic events. Several other SERMs are in development to improve tissue specificity, efficacy and tolerance. Raloxifene shows protection against vertebral fractures similar to bisphosphonates; however, no significant effect has been observed on nonvertebral fractures. Many SERMs are in development for prevention and treatment of osteoporosis. As breast cancer metastasizes early and advanced disease cannot be cured, prevention is essential. To avoid the concerns about the use of traditional hormone replacement therapy, dehydroepiandrosterone—a tissue-targeted precursor of sex steroid formation—offers hope of a physiological tissue-targeted hormone replacement that, combined with a SERM, would simultaneously prevent breast and uterine cancer.
Key Points
-
The selective estrogen receptor modulator (SERM) tamoxifen blocks estrogen action and prolongs survival in breast cancer
-
New SERMs have more potent activity in the mammary gland and at least one does not stimulate the uterus, reducing the risk of uterine cancer
-
The best hope of decreasing death from breast cancer is prevention of the disease, thereby avoiding micrometastases
-
Dehydroepiandrosterone (DHEA) is the precursor component of tissue-specific hormone replacement therapy (HRT) and avoids exposure of other tissues as found with traditional HRT
-
DHEA allows compensation for the loss of androgens at menopause exclusively in the tissues that need them
-
The addition of a highly specific SERM to DHEA might offer the best hope to prevent breast and uterine cancer and to replace traditional HRT, thereby meeting many other needs of women after the menopause
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Jemal A et al. (2007) Cancer statistics, 2007. CA Cancer J Clin 57: 43–66
Berry DA et al. (2005) Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 353: 1784–1792
Fisher B et al. (1996) Five versus more than five years of tamoxifen for breast cancer patients with negative lymph nodes and estrogen positive tumors. J Natl Cancer Inst 88: 1529–1542
Early Breast Cancer Trialists' Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365: 1687–1717
Women's Health Initiative (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women. JAMA 288: 321–333
Beral V et al. (2005) Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet 365: 1543–1551
Archer DF et al. (1999) A randomized comparison of continuous combined transdermal delivery of estradiol-norethindrone acetate and estradiol alone for menopause. CombiPatch Study Group. Obstet Gynecol 94: 498–503
Labrie F et al. (2001) EM-652 (SCH 57068), a pure SERM having complete antiestrogenic activity in the mammary gland and endometrium. J Steroid Biochem Mol Biol 79: 213–225
Vogel VG et al. (2006) Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 295: 2727–2741
Barrett-Connor E et al. (2006) Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 355: 125–137
Melnikow J et al. (2006) Chemoprevention: drug pricing and mortality: the case of tamoxifen. Cancer 107: 950–958
Vogel VG et al. (2002) Re: tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 94: 1504
Land SR et al. (2006) Patient-reported symptoms and quality of life during treatment with tamoxifen or raloxifene for breast cancer prevention: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 295: 2742–2751
Paridaens R et al. (2003) Mature results of a randomized phase II multicenter study of exemestane versus tamoxifen as first-line hormone therapy for postmenopausal women with metastatic breast cancer. Ann Oncol 14: 1391–1398
Coombes RC et al. (2004) A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 350: 1081–1092
Poulin R and Labrie F (1986) Stimulation of cell proliferation and estrogenic response by adrenal C19-Δ5-steroids in the ZR-75-1 human breast cancer cell line. Cancer Res 46: 4933–4937
Howell A et al. (2005) Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet 365: 60–62
Howell A et al. (2004) Comparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: a multinational, double-blind, randomized trial. J Clin Oncol 22: 1605–1613
Howell A et al. (2002) Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progression after prior endocrine treatment. J Clin Oncol 20: 3396–3403
Johnston SR (2005) Endocrinology and hormone therapy in breast cancer: selective oestrogen receptor modulators and downregulators for breast cancer—have they lost their way? Breast Cancer Res 7: 119–130
Roy J et al. (2003) A novel pure SERM achieves complete regression of the majority of human breast cancer tumors in nude mice. Breast Cancer Res Treat 81: 223–229
Gutman M et al. (2002) Comparison of the effects of EM-652 (SCH 57068), tamoxifen, toremifene, droloxifene, idoxifene, GW-5638 and raloxifene on the growth of human ZR-75-1 breast tumors in nude mice. Int J Cancer 99: 273–278
Gutman M et al. (2003) Effect of treatment sequence with radiotherapy and the antiestrogen EM 800 on the growth of ZR 75 1 human mammary carcinoma in nude mice. Int J Cancer 103: 268–276
Simard J et al. (1997) Blockade of the stimulatory effect of estrogens, OH-tamoxifen, OH-toremifene, droloxifene and raloxifene on alkaline phosphatase activity by the antiestrogen EM-800 in human endometrial adenocarcinoma Ishikawa cells. Cancer Res 57: 3494–3497
Labrie F et al. (2004) Activity and safety of the orally active pure antiestrogen EM-800 (SCH 57050) in tamoxifen-resistant breast cancer. J Clin Oncol 22: 864–871
Duvernoy CS et al. (2005) Vascular events in the multiple outcomes of raloxifene evaluation (MORE) trial: incidence, patient characteristics and effect of raloxifene. Menopause 12: 444–452
Labrie F et al. (2005) Is DHEA a hormone? Starling Review. J Endocrinol 187: 169–196
Labrie F et al. (2006) Androgen glucuronides, instead of testosterone as the new markers of androgenic activity in women. J Steroid Biochem Mol Biol 99: 182–188
Wierman ME et al. (2006) Androgen therapy in women: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 91: 3697–3710
Gammon MD and Thompson WD (1991) Polycystic ovaries and the risk of breast cancer. Am J Epidemiol 134: 818–824
Dimitrakakis C et al. (2003) A physiologic role for testosterone in limiting estrogenic stimulation of the breast. Menopause 10: 292–298
Labrie F et al. (2003) Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr Rev 24: 152–182
Ulrich P (1939) Testosterone (male hormone) and its possible role in the treatment of some breast cancers [French]. Acta - Unio Internationalis Contra Cancrum 4: 377–379
Sturgeon SR et al. (2004) Serum levels of sex hormones and breast cancer risk in premenopausal women: a case-control study (USA). Cancer Causes Control 15: 45–53
Key T et al. (2002) Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst 94: 606–616
Beattie MS et al. (2006) Endogenous sex hormones, breast cancer risk, and tamoxifen response: an ancillary study in the NSABP Breast Cancer Prevention Trial (P-1). J Natl Cancer Inst 98: 110–115
Onland-Moret NC et al. (2003) Urinary endogenous sex hormone levels and the risk of postmenopausal breast cancer. Br J Cancer 88: 1394–1399
Tworoger SS et al. (2006) The association of plasma DHEA and DHEA sulfate with breast cancer risk in predominantly premenopausal women. Cancer Epidemiol Biomarkers Prevent 15: 967–971
Tamimi RM et al. (2006) Combined estrogen and testosterone use and risk of breast cancer in postmenopausal women. Arch Intern Med 166: 1483–1489
Vermeulen A et al. (1986) Aromatase, 17β-hydroxysteroid dehydrogenase and intratissular sex hormone concentrations in cancerous and normal glandular breast tissue in postmenopausal women. J Steroid Biochem 25: 799–802
Bone Health and Osteoporosis: A Report of the Surgeon General [http://www.surgeongeneral.gov/library/bonehealth/] (accessed March 27 2007)
Fisher B et al. (1998) Tamoxifen for prevention of breast cancer: report of the national surgical adjuvant breast and bowel project P-1 study. J Natl Cancer Inst 90: 1371–1388
Delmas PD et al. (2002) Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: four-year results from a randomized clinical trial. J Clin Endocrinol Metab 87: 3609–3617
Ettinger B et al. (2004) Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res 19: 745–751
Michalska D et al. (2006) The effect of raloxifene after discontinuation of long-term alendronate treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab 91: 870–877
Martel C et al. (1998) Predominant androgenic component in the stimulatory effect of dehydroepiandrosterone on bone mineral density in the rat. J Endocrinol 157: 433–442
Labrie F et al. (1997) Effect of 12-month DHEA replacement therapy on bone, vagina, and endometrium in postmenopausal women. J Clin Endocrinol Metab 82: 3498–3505
Diamond P et al. (1996) Metabolic effects of 12-month percutaneous dehydroepiandrosterone replacement therapy in postmenopausal women. J Endocrinol Suppl 150: S43–S50
Villareal DT and Holloszy JO (2004) Effect of DHEA on abdominal fat and insulin action on elderly women and men: a randomized controlled trial. JAMA 292: 2243–2248
Morales AJ et al. (1998) The effect of six months treatment with a 100 mg daily dose of dehydroepiandrosterone (DHEA) on circulating sex steroids, body composition and muscle strength in age-advanced men and women. Clin Endocrinol 49: 421–432
Jankowski CM et al. (2006) Effects of dehydroepiandrosterone replacement therapy on bone mineral density in older adults: a randomized, controlled trial. J Clin Endocrinol Metab 91: 2986–2993
Nair KS et al. (2006) DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med 355: 1647–1659
Weigelt B et al. (2003) Gene expression profiles of primary breast tumors maintained in distant metastases. Proc Natl Acad Sci USA 100: 15901–15905
Labrie F et al. (2003) The combination of a novel SERM with an estrogen protects the mammary gland and uterus in a rodent model: the future of postmenopausal women's health? Endocrinology 144: 4700–4706
Stomati M et al. (2000) Six-month oral dehydroepiandrosterone supplementation in early and late postmenopause. Gynecol Endocrinol 14: 342–36355
Author information
Authors and Affiliations
Ethics declarations
Competing interests
F Labrie is President of Endorecherche, Quebec City, QC, Canada, the company developing acolbifene and dehydroepiandrosterone.
Rights and permissions
About this article
Cite this article
Labrie, F. Drug Insight: breast cancer prevention and tissue-targeted hormone replacement therapy. Nat Rev Endocrinol 3, 584–593 (2007). https://doi.org/10.1038/ncpendmet0559
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpendmet0559
This article is cited by
-
Chondroitin sulfate-E mediates estrogen-induced osteoanabolism
Scientific Reports (2015)
-
Knockdown of ANLN by lentivirus inhibits cell growth and migration in human breast cancer
Molecular and Cellular Biochemistry (2015)
-
Distinct patterns of promoter CpG island methylation of breast cancer subtypes are associated with stem cell phenotypes
Modern Pathology (2012)
-
Specific transcriptional response of four blockers of estrogen receptors on estradiol-modulated genes in the mouse mammary gland
Breast Cancer Research and Treatment (2012)