Abstract
Epidemiological studies have shown that age is the chief risk factor for atherosclerotic cardiovascular diseases, but the molecular mechanisms that underlie the increase in risk conferred by aging remain unclear. Evidence suggests that the cardiovascular repair system is impaired with advancing age, thereby inducing age-associated cardiovascular dysfunction. Such impairment could be attributable to senescence of cardiovascular tissues at the cellular level as a result of telomere shortening, DNA damage, and genomic instability. In fact, the replicative ability of cardiovascular cells, particularly stem cells and/or progenitor cells, has been shown to decline with age. Recently, considerable progress has been made in understanding the pathogenesis of human progeroid syndromes that feature cardiovascular aging. Most of the genes responsible have a role in DNA metabolism, and mutated forms of these genes result in alterations of the response to DNA damage and in decreased cell proliferation, which might be common features of a phenotype of aging. Here we review the cardiovascular research on cellular senescence, stem cell aging, and progeroid syndromes and discuss the potential role of cellular senescence in the mechanisms underlying both normal aging and premature aging syndromes.
Key Points
-
Cellular senescence probably contributes to the pathogenesis of cardiovascular disease
-
Telomere integrity is impaired with advancing age, which leads to vascular dysfunction
-
The number and function of cardiovascular stem cells and/or progenitor cells shows a progressive decline with age
-
Telomere attrition, DNA damage, and genomic instability might be common features of both normal aging and progeroid syndromes
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lakatta EG and Levy D (2003) Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107: 139–146
Lakatta EG (2003) Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation 107: 490–497
Reed MJ and Edelberg JM (2004) Impaired angiogenesis in the aged. Sci Aging Knowledge Environ 7: pe7
Gennaro G et al. (2003) Age-dependent impairment of reendothelialization after arterial injury: role of vascular endothelial growth factor. Circulation 107: 230–233
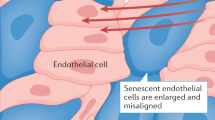
Minamino T and Komuro I (2007) Vascular cell senescence: contribution to atherosclerosis. Circ Res 100: 15–26
Ballard VL and Edelberg JM (2007) Stem cells and the regeneration of the aging cardiovascular system. Circ Res 100: 1116–1127
Thompson KV and Holliday R (1983) Genetic effects on the longevity of cultured human fibroblasts: II. DNA repair deficient syndromes. Gerontology 29: 83–88
Burrig KF (1991) The endothelium of advanced arteriosclerotic plaques in humans. Arterioscler Thromb 11: 1678–1689
Ross R et al. (1984) Human atherosclerosis: I. cell constitution and characteristics of advanced lesions of the superficial femoral artery. Am J Pathol 114: 79–93
Minamino T et al. (2002) Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 105: 1541–1544
Minamino T et al. (2003) Ras induces vascular smooth muscle cell senescence and inflammation in human atherosclerosis. Circulation 108: 2264–2269
Blasco MA (2005) Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet 6: 611–622
Herbig U et al. (2004) Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol Cell 14: 501–513
d'Adda di Fagagna F et al. (2003) A DNA damage checkpoint response in telomere-initiated senescence. Nature 426: 194–198
Takai H et al. (2003) DNA damage foci at dysfunctional telomeres. Curr Biol 13: 1549–1556
Herbig U et al. (2006) Cellular senescence in aging primates. Science 311: 1257
Chang E and Harley CB (1995) Telomere length and replicative aging in human vascular tissues. Proc Natl Acad Sci USA 92: 11190–11194
Aviv H et al. (2001) Age dependent aneuploidy and telomere length of the human vascular endothelium. Atherosclerosis 159: 281–287
Ogami M et al. (2004) Telomere shortening in human coronary artery diseases. Arterioscler Thromb Vasc Biol 24: 546–550
Voghel G et al. (2007) Cellular senescence in endothelial cells from atherosclerotic patients is accelerated by oxidative stress associated with cardiovascular risk factors. Mech Ageing Dev 128: 662–671
Minamino T and Kourembanas S (2001) Mechanisms of telomerase induction during vascular smooth muscle cell proliferation. Circ Res 89: 237–243
Minamino T et al. (2001) Hypoxia extends the life span of vascular smooth muscle cells through telomerase activation. Mol Cell Biol 21: 3336–3342
Matsushita H et al. (2001) eNOS activity is reduced in senescent human endothelial cells: preservation by hTERT immortalization. Circ Res 89: 793–798
Yang J et al. (1999) Human endothelial cell life extension by telomerase expression. J Biol Chem 274: 26141–26148
Yang J et al. (2001) Telomerized human microvasculature is functional in vivo. Nat Biotechnol 19: 219–224
Blasco MA et al. (1997) Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91: 25–34
Lee HW et al. (1998) Essential role of mouse telomerase in highly proliferative organs. Nature 392: 569–574
Rudolph KL et al. (1999) Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell 96: 701–712
Franco S et al. (2002) Decreased B16F10 melanoma growth and impaired vascularization in telomerase-deficient mice with critically short telomeres. Cancer Res 62: 552–559
Poch E et al. (2004) Short telomeres protect from diet-induced atherosclerosis in apolipoprotein E-null mice. FASEB J 18: 418–420
Perez-Rivero G et al. (2006) Mice deficient in telomerase activity develop hypertension because of an excess of endothelin production. Circulation 114: 309–317
Najjar SS et al. (2005) Arterial aging: is it an immutable cardiovascular risk factor? Hypertension 46: 454–462
Kunieda T et al. (2006) Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation 114: 953–960
Matthews C et al. (2006) Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circ Res 99: 156–164
Kurz DJ et al. (2004) Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci 117: 2417–2426
Voghel G et al. (2008) Chronic treatment with N-acetyl-cystein delays cellular senescence in endothelial cells isolated from a subgroup of atherosclerotic patients. Mech Ageing Dev 129: 261–270
Hasty P et al. (2003) Aging and genome maintenance: lessons from the mouse? Science 299: 1355–1359
Tyner SD et al. (2002) p53 mutant mice that display early ageing-associated phenotypes. Nature 415: 45–53
Maier B et al. (2004) Modulation of mammalian life span by the short isoform of p53. Genes Dev 18: 306–319
Cao L et al. (2003) Senescence, aging, and malignant transformation mediated by p53 in mice lacking the Brca1 full-length isoform. Genes Dev 17: 201–213
Asahara T et al. (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–967
Shi Q et al. (1998) Evidence for circulating bone marrow-derived endothelial cells. Blood 92: 362–367
Rafii S and Lyden D (2003) Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med 9: 702–712
Walter DH et al. (2002) Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation 105: 3017–3024
Purhonen S et al. (2008) Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci USA 105: 6620–6625
Thum T et al. (2007) Age-dependent impairment of endothelial progenitor cells is corrected by growth-hormone-mediated increase of insulin-like growth-factor-1. Circ Res 100: 434–443
Vasa M et al. (2001) Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 89: E1–E7
Scheubel RJ et al. (2003) Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol 42: 2073–2080
Hill JM et al. (2003) Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 348: 593–600
Sugihara S et al. (2007) Age-related BM-MNC dysfunction hampers neovascularization. Mech Ageing Dev 128: 511–516
Heeschen C et al. (2004) Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation 109: 1615–1622
Ward M R et al. (2007) Endothelial progenitor cell therapy for the treatment of coronary disease, acute MI, and pulmonary arterial hypertension: current perspectives. Catheter Cardiovasc Interv 70: 983–998
Edelberg JM et al. (2002) Young adult bone marrow-derived endothelial precursor cells restore aging-impaired cardiac angiogenic function. Circ Res 90: E89–E93
Rauscher FM et al. (2003) Aging, progenitor cell exhaustion, and atherosclerosis. Circulation 108: 457–463
Wassmann S et al. (2006) Improvement of endothelial function by systemic transfusion of vascular progenitor cells. Circ Res 99: e74–e83
Werner N et al. (2005) Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 353: 999–1007
Samani NJ et al. (2001) Telomere shortening in atherosclerosis. Lancet 358: 472–473
Satoh M et al. (2008) Association between oxidative DNA damage and telomere shortening in circulating endothelial progenitor cells obtained from metabolic syndrome patients with coronary artery disease. Atherosclerosis [10.1016/j.atherosclerosis.2007.09.040]
Rosso A et al. (2006) p53 Mediates the accelerated onset of senescence of endothelial progenitor cells in diabetes. J Biol Chem 281: 4339–4347
Shantsila, E et al. (2007) Endothelial progenitor cells in cardiovascular disorders. J Am Coll Cardiol 49: 741–752
Zhu JH et al. (2006) Homocysteine accelerates senescence and reduces proliferation of endothelial progenitor cells. J Mol Cell Cardiol 40: 648–652
Murasawa S et al. (2002) Constitutive human telomerase reverse transcriptase expression enhances regenerative properties of endothelial progenitor cells. Circulation 106: 1133–1139
Spyridopoulos I et al. (2004) Statins enhance migratory capacity by upregulation of the telomere repeat-binding factor TRF2 in endothelial progenitor cells. Circulation 110: 3136–3142
Assmus B et al. (2003) HMG-CoA reductase inhibitors reduce senescence and increase proliferation of endothelial progenitor cells via regulation of cell cycle regulatory genes. Circ Res 92: 1049–1055
Bosch-Marce M et al. (2007) Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circ Res 101: 1310–1318
Chang EI et al. (2007) Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1alpha stabilization during ischemia. Circulation 116: 2818–2829
Martin GM (2005) Genetic modulation of senescent phenotypes in Homo sapiens. Cell 120: 523–532
Capell B C et al. (2007) Mechanisms of cardiovascular disease in accelerated aging syndromes. Circ Res 101: 13–26
Hennekam RC (2006) Hutchinson–Gilford progeria syndrome: review of the phenotype. Am J Med Genet A 140: 2603–2624
Stehbens WE et al. (1999) Histological and ultrastructural features of atherosclerosis in progeria. Cardiovasc Pathol 8: 29–39
De Sandre-Giovannoli A et al. (2003) Lamin A truncation in Hutchinson–Gilford progeria. Science 300: 2055
Eriksson M et al. (2003) Recurrent de novo point mutations in lamin A cause Hutchinson–Gilford progeria syndrome. Nature 423: 293–298
Mounkes LC et al. (2003) A progeroid syndrome in mice is caused by defects in A-type lamins. Nature 423: 298–301
Varga R et al. (2006) Progressive vascular smooth muscle cell defects in a mouse model of Hutchinson–Gilford progeria syndrome. Proc Natl Acad Sci USA 103: 3250–3255
Bergo MO et al. (2002) Zmpste24 deficiency in mice causes spontaneous bone fractures, muscle weakness, and a prelamin A processing defect. Proc Natl Acad Sci USA 99: 13049–13054
Fong LG et al. (2004) Heterozygosity for Lamin A deficiency eliminates the progeria-like phenotypes in Zmpste24-deficient mice. Proc Natl Acad Sci USA 101: 18111–18116
Yang SH et al. (2005) Blocking protein farnesyltransferase improves nuclear blebbing in mouse fibroblasts with a targeted Hutchinson–Gilford progeria syndrome mutation. Proc Natl Acad Sci USA 102: 10291–10296
Varela I et al. (2008) Combined treatment with statins and aminobisphosphonates extends longevity in a mouse model of human premature aging. Nat Med 14: 767–772
Shumaker DK et al. (2006) Mutant nuclear lamin A leads to progressive alterations of epigenetic control in premature aging. Proc Natl Acad Sci USA 103: 8703–8708
Goldman RD et al. (2004) Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson–Gilford progeria syndrome. Proc Natl Acad Sci USA 101: 8963–8968
Liu B et al. (2005) Genomic instability in laminopathy-based premature aging. Nat Med 11: 780–785
Varela I et al. (2005) Accelerated ageing in mice deficient in Zmpste24 protease is linked to p53 signalling activation. Nature 437: 564–568
Scaffidi P and Misteli T (2008) Lamin A-dependent misregulation of adult stem cells associated with accelerated ageing. Nat Cell Biol 10: 452–459
Halaschek-Wiener J and Brooks-Wilson A (2007) Progeria of stem cells: stem cell exhaustion in Hutchinson–Gilford progeria syndrome. J Gerontol A Biol Sci Med Sci 62: 3–8
Cohen JI et al. (1987) Cardiovascular features of the Werner syndrome. Am J Cardiol 59: 493–495
Yu CE et al. (1996) Positional cloning of the Werner's syndrome gene. Science 272: 258–262
Opresko PL et al. (2004) Junction of RecQ helicase biochemistry and human disease. J Biol Chem 279: 18099–18102
Lombard DB et al. (2000) Mutations in the WRN gene in mice accelerate mortality in a p53-null background. Mol Cell Biol 20: 3286–3291
Wang L et al. (2000) Cellular Werner phenotypes in mice expressing a putative dominant-negative human WRN gene. Genetics 154: 357–362
Lebel M and Leder P (1998) A deletion within the murine Werner syndrome helicase induces sensitivity to inhibitors of topoisomerase and loss of cellular proliferative capacity. Proc Natl Acad Sci USA 95: 13097–13102
Chang S et al. (2004) Essential role of limiting telomeres in the pathogenesis of Werner syndrome. Nat Genet 36: 877–882
Du X et al. (2004) Telomere shortening exposes functions for the mouse Werner and Bloom syndrome genes. Mol Cell Biol 24: 8437–8446
Wyllie FS et al. (2000) Telomerase prevents the accelerated cell ageing of Werner syndrome fibroblasts. Nat Genet 24: 16–17
Crabbe L et al. (2007) Telomere dysfunction as a cause of genomic instability in Werner syndrome. Proc Natl Acad Sci USA 104: 2205–2210
Massip L et al. (2006) Increased insulin, triglycerides, reactive oxygen species, and cardiac fibrosis in mice with a mutation in the helicase domain of the Werner syndrome gene homologue. Exp Gerontol 41: 157–168
Ogawa D et al. (2006) Activation of peroxisome proliferator-activated receptor gamma suppresses telomerase activity in vascular smooth muscle cells. Circ Res 98: e50–e59
Bode-Boger SM et al. (2005) Aspirin reduces endothelial cell senescence. Biochem Biophys Res Commun 334: 1226–1232
Komarov PG et al. (1999) A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science 285: 1733–1737
Russell SJ and Kahn CR (2007) Endocrine regulation of ageing. Nat Rev Mol Cell Biol 8: 681–691
Krishnamurthy J et al. (2004) Ink4a/Arf expression is a biomarker of aging. J Clin Invest 114: 1299–1307
Acknowledgements
T Minamino was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by grants from the Suzuken Memorial Foundation, the Japan Diabetes Foundation, the Ichiro Kanehara Foundation, the Tokyo Biochemical Research Foundation, and the Takeda Science Foundation. I Komuro was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture and by Health and Labor Sciences Research grants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Minamino, T., Komuro, I. Vascular aging: insights from studies on cellular senescence, stem cell aging, and progeroid syndromes. Nat Rev Cardiol 5, 637–648 (2008). https://doi.org/10.1038/ncpcardio1324
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncpcardio1324
This article is cited by
-
Aging-associated inflammation and fibrosis in arachnoid membrane
BMC Neurology (2021)
-
CD9 induces cellular senescence and aggravates atherosclerotic plaque formation
Cell Death & Differentiation (2020)
-
Hutchinson–Gilford progeria syndrome as a model for vascular aging
Biogerontology (2016)
-
Mitochondria and oxidative stress in heart aging
AGE (2016)
-
Gestational diabetes induces alterations in the function of neonatal endothelial colony-forming cells
Pediatric Research (2014)