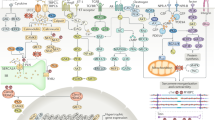
Abstract
Acute decompensated heart failure (ADHF) is responsible for more than 1 million hospital admissions each year in the US. Clinicians and scientists have developed therapeutic strategies that reduce mortality in patients with chronic heart failure (HF). Despite the widely appreciated magnitude of the ADHF problem, there is still a critical gap in our understanding of the cellular mechanisms involved and effective treatment strategies for hospitalized patients. Irrespective of the etiology, patients with ADHF present with similar symptoms (e.g. edema, altered hemodynamics and congestion) as multiple signaling pathways converge in a common phenotypic presentation. Investigations have shown that patients with ADHF have increased catecholamine levels, which cause chronic stimulation of β-adrenergic receptors. This overstimulation leads to chronic G-protein activation and perturbations in myocyte signaling, as the patient's heart attempts to adapt to progressive HF. Over time, these compensatory signaling mechanisms ultimately fail, and maladaptive signaling prevails with progressive worsening of symptoms. This Review summarizes some of the changes that occur during chronic adrenergic stimulation, and examines how downstream contractile dysfunction and myocyte death can alter the prognosis of patients with HF hospitalized for acute events.
Key Points
-
Acute decompensated heart failure (ADHF) is a large clinical problem, yet little is known about the basic mechanisms associated with disease progression; the final common pathway in ADHF is increased adrenergic signaling, independent of ADHF etiology
-
Long-term (or maladaptive) adrenergic signaling in ADHF will lead to activation of calcium/calmodulin-dependent protein kinase, increased cytokine levels, nitroso–redox imbalance, and a shift in Ca2+ pools from the sarcoplasmic reticulum to the cytosol
-
Maladaptive adrenergic signaling leads to caspase activation, cytochrome c translocation and progressive cell death, leading in turn to progressive worsening of heart function
-
Maladaptive adrenergic signaling alters cardiac excitation–contraction coupling, which leads to decreased contractility and an increased propensity for arrhythmias
-
Therapeutic targeting of these two common pathways (cell death and altered excitation–contraction coupling) of ADHF may improve patient recovery
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Heart Failure Society of America (2006) HFSA 2006 Comprehensive heart failure practice guideline. J Card Fail 12: e1–2
Tilley DG and Rockman HA (2006) Role of β-adrenergic receptor signaling and desensitization in heart failure: new concepts and prospects for treatment. Expert Rev Cardiovasc Ther 4: 417–432
Feldman DS et al. (2005) Mechanisms of disease: β-adrenergic receptors—alterations in signal transduction and pharmacogenomics in heart failure. Nat Clin Pract Cardiovasc Med 2: 475–483
Jong P et al. (2002) Prognosis and determinants of survival in patients newly hospitalized for heart failure: a population-based study. Arch Intern Med 162: 1689–1694
Feldman D et al. (2007) Management strategies for stage-d patients with acute heart failure. Clin Cardiol [10.1002/clc.20251]
Doughty RN et al. (1997) Left ventricular remodeling with carvedilol in patients with congestive heart failure due to ischemic heart disease. Australia–New Zealand Heart Failure Research Collaborative Group. J Am Coll Cardiol 29: 1060–1066
CIBIS-II Investigators and Committees (1999) The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 353: 9–13
MERIT-HF Study Group (1999) Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 353: 2001–2007
Packer M et al. (1996) The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. US Carvedilol Heart Failure Study Group. N Engl J Med 334: 1349–1355
Lohse MJ et al. (2003) What is the role of β-adrenergic signaling in heart failure. Circ Res 93: 896–906
Vatner SF et al. (2000) β-Adrenergic receptor signaling: an acute compensatory adjustment-inappropriate for the chronic stress of heart failure? Insights from Gsalpha overexpression and other genetically engineered animal models. Circ Res 86: 502–506
Bristow MR et al. (1990) β-Adrenergic pathways in nonfailing and failing human ventricular myocardium. Circulation 82 (2 Suppl): I12–I25
Rajagopal K et al. (2006) β-Arrestin2-mediated inotropic effects of the angiotensin II type 1A receptor in isolated cardiac myocytes. Proc Natl Acad Sci USA 103: 16284–16289
Noma T et al. (2007) β-Arrestin-mediated β1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest 117: 2445–2458
Ojeda S et al. (2005) Short- and long-term results of a programme for the prevention of readmissions and mortality in patients with heart failure: are effects maintained after stopping the programme? Eur J Heart Fail 7: 921–926
Lefkowitz RJ (1993) G protein-coupled receptor kinases. Cell 74: 409–412
Iaccarino G et al. (2005) Elevated myocardial and lymphocyte GRK2 expression and activity in human heart failure. Eur Heart J 26: 1752–1758
Luttrell LM et al. (1999) β-Arrestin-dependent formation of β2 adrenergic receptor-Src protein kinase complexes. Science 283: 655–661
Dent MR et al. (2007) Alterations in both death and survival signals for apoptosis in heart failure due to volume overload. J Mol Cell Cardiol 43: 726–732
Muraski JA et al. (2007) Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat Med 13: 1467–1475
Singh K et al. (2001) Adrenergic regulation of cardiac myocyte apoptosis. J Cell Physiol 189: 257–265
Beltrami CA et al. (1994) Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation 89: 151–163
Communal C and Colucci WS (2005) The control of cardiomyocyte apoptosis via the β-adrenergic signaling pathways. Arch Mal Coeur Vaiss 98: 236–241
Wencker D et al. (2003) A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest 111: 1497–1504
Xiang Y and Kobilka BK (2003) Myocyte adrenoceptor signaling pathways. Science 300: 1530–1532
Beltrami CA et al. (1995) The cellular basis of dilated cardiomyopathy in humans. J Mol Cell Cardiol 27: 291–305
Olivetti G et al. (1997) Apoptosis in the failing human heart. N Engl J Med 336: 1131–1141
Narula J et al. (1996) Apoptosis in myocytes in end-stage heart failure. N Engl J Med 335: 1182–1189
Rossig L et al. (2000) Congestive heart failure induces endothelial cell apoptosis: protective role of carvedilol. J Am Coll Cardiol 36: 2081–2089
Chang J et al. (2006) Activation of Rho-associated coiled-coil protein kinase 1 (ROCK-1) by caspase-3 cleavage plays an essential role in cardiac myocyte apoptosis. Proc Natl Acad Sci USA 103: 14495–14500
Cardone MH et al. (1998) Regulation of cell death protease caspase-9 by phosphorylation. Science 282: 1318–1321
Mallat Z et al. (1996) Evidence of apoptosis in arrhythmogenic right ventricular dysplasia. N Engl J Med 335: 1190–1196
Bing OH (1994) Hypothesis: apoptosis may be a mechanism for the transition to heart failure with chronic pressure overload. J Mol Cell Cardiol 26: 943–948
Kitsis RN and Mann DL (2005) Apoptosis and the heart: a decade of progress. J Mol Cell Cardiol 38: 1–2
Communal C et al. (2002) Functional consequences of caspase activation in cardiac myocytes. Proc Natl Acad Sci USA 99: 6252–6256
Narula J et al. (2001) Apoptosis and the systolic dysfunction in congestive heart failure: story of apoptosis interruptus and zombie myocytes. Cardiol Clin 19: 113–126
Chandrashekhar Y et al. (2004) Long-term caspase inhibition ameliorates apoptosis, reduces myocardial troponin-I cleavage, protects left ventricular function, and attenuates remodeling in rats with myocardial infarction. J Am Coll Cardiol 43: 295–301
Laugwitz KL et al. (2001) Blocking caspase-activated apoptosis improves contractility in failing myocardium. Hum Gene Ther 12: 2051–2063
Bers DM (2001) Excitation-Contraction Coupling and Cardiac Contractile Force. Dordrecht: Kluwer Academic Publishers
Ziolo MT et al. (2005) Adenoviral gene transfer of mutant phospholamban rescues contractile dysfunction in failing rabbit myocytes with relatively preserved SERCA function. Circ Res 96: 815–817
Piacentino V III et al. (2003) Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res 92: 651–658
Maier LS et al. (2006) Dynamic changes in free Ca–calmodulin levels in adult cardiac myocytes. J Mol Cell Cardiol 41: 451–458
Curran J et al. (2007) β-Adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ Res 100: 391–398
Kohlhaas M et al. (2006) Increased sarcoplasmic reticulum calcium leak but unaltered contractility by acute CaMKII overexpression in isolated rabbit cardiac myocytes. Circ Res 98: 235–244
Bassani JW et al. (1995) Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol 268: C1313–C1319
Pogwizd SM et al. (2001) Arrhythmogenesis and contractile dysfunction in heart failure: roles of sodium-calcium exchange, inward rectifier potassium current, and residual β-adrenergic responsiveness. Circ Res 88: 1159–1167
Venetucci LA et al. (2007) Increasing ryanodine receptor open probability alone does not produce arrhythmogenic calcium waves: threshold sarcoplasmic reticulum calcium content is required. Circ Res 100: 105–111
Wagner S et al. (2006) Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest 116: 3127–3138
Olshansky B et al. (2007) Where patients with mild to moderate heart failure die: results from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Am Heart J 153: 1089–1094
Srivastava S et al. (2007) Downregulation of CuZn-superoxide dismutase contributes to β-adrenergic receptor-mediated oxidative stress in the heart. Cardiovasc Res 74: 445–455
Baumgarten G et al (2002) Load-dependent and -independent regulation of proinflammatory cytokine and cytokine receptor gene expression in the adult mammalian heart. Circulation 105: 2192–2197
Niebauer J et al. (1999) Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet 353: 1838–1842
Suzuki H et al. (2005) Time-course of changes in the levels of interleukin 6 in acutely decompensated heart failure. Int J Cardiol 100: 415–420
Csont T et al. (2005) The involvement of superoxide and iNOS-derived NO in cardiac dysfunction induced by pro-inflammatory cytokines. J Mol Cell Cardiol 39: 833–840
Persad S et al. (1998) Modification of cardiac β-adrenoceptor mechanisms by H2O2 . Am J Physiol 274: H416–H423
Zeitz O et al. (2002) Hydroxyl radical-induced acute diastolic dysfunction is due to calcium overload via reverse-mode Na(+)-Ca(2+) exchange. Circ Res 90: 988–995
Xu KY et al. (1997) Hydroxyl radical inhibits sarcoplasmic reticulum Ca(2+)-ATPase function by direct attack on the ATP binding site. Circ Res 80: 76–81
Goldhaber JI (1996) Free radicals enhance Na+/Ca2+ exchange in ventricular myocytes. Am J Physiol 271: H823–H833
Zima AV et al. (2004) Effects of cytosolic NADH/NAD(+) levels on sarcoplasmic reticulum Ca(2+) release in permeabilized rat ventricular myocytes. J Physiol 555: 727–741
Song Y et al. (2006) Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther 318: 214–222
Ziolo MT and Bers DM (2003) The real estate of NOS signaling: location, location, location. Circ Res 92: 1279–1281
Ziolo MT et al. (2001) Myocytes isolated from rejecting transplanted rat hearts exhibit a nitric oxide-mediated reduction in the calcium current. J Mol Cell Cardiol 33: 1691–1699
Hu A et al. (2006) Chronic β-adrenergic receptor stimulation induces cardiac apoptosis and aggravates myocardial ischemia/reperfusion injury by provoking inducible nitric-oxide synthase-mediated nitrative stress. J Pharmacol Exp Ther 318: 469–475
Oddis CV et al. (1995) cAMP enhances inducible nitric oxide synthase mRNA stability in cardiac myocytes. Am J Physiol 269: H2044–H2050
Ziolo MT et al. (2001) Expression of inducible nitric oxide synthase depresses β-adrenergic-stimulated calcium release from the sarcoplasmic reticulum in intact ventricular myocytes. Circulation 104: 2961–2966
Ziolo MT et al. (2004) Myocyte nitric oxide synthase 2 contributes to blunted β-adrenergic response in failing human hearts by decreasing Ca2+ transients. Circulation 109: 1886–1891
Kohr MJ et al.: Targeting of phospholamban by peroxynitrite decreases β-adrenergic stimulation in cardiomyocytes. Cardiovasc Res, in press
Adam L et al. (1999) Nitric oxide modulates β(2)-adrenergic receptor palmitoylation and signaling. J Biol Chem 274: 26337–26343
Saraiva RM et al. (2005) Deficiency of neuronal nitric oxide synthase increases mortality and cardiac remodeling after myocardial infarction: role of nitroso-redox equilibrium. Circulation 112: 3415–3422
Buys ES et al. (2007) Cardiomyocyte-restricted restoration of nitric oxide synthase 3 attenuates left ventricular remodeling after chronic pressure overload. Am J Physiol Heart Circ Physiol 293: H620–H627
Damy T et al. (2004) Increased neuronal nitric oxide synthase-derived NO production in the failing human heart. Lancet 363: 1365–1367
Bendall JK et al. (2004) Role of myocardial neuronal nitric oxide synthase-derived nitric oxide in β-adrenergic hyporesponsiveness after myocardial infarction-induced heart failure in rat. Circulation 110: 2368–2375
Whalen EJ et al. (2007) Regulation of β-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell 129: 511–522
Hare JM and Stamler JS (2005) NO/redox disequilibrium in the failing heart and cardiovascular system. J Clin Invest 115: 509–517
Kubalova Z et al. (2005) Abnormal intrastore calcium signaling in chronic heart failure. Proc Natl Acad Sci USA 102: 14104–14109
Nishijima Y et al. (2007) Chronic cardiac resynchronization therapy and reverse ventricular remodeling in a model of nonischemic cardiomyopathy. Life Sci 81: 1152–1159
Acknowledgements
We thank Dr Jonathan Davis for critically reading the manuscript. Dr TS Elton (R01 HL048848), Dr DS Feldman (R01 HL084498), and Dr MT Ziolo (R01 HL079283) are supported by the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Feldman, D., Elton, T., Sun, B. et al. Mechanisms of Disease: detrimental adrenergic signaling in acute decompensated heart failure. Nat Rev Cardiol 5, 208–218 (2008). https://doi.org/10.1038/ncpcardio1127
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncpcardio1127
This article is cited by
-
Rationally engineered Troponin C modulates in vivo cardiac function and performance in health and disease
Nature Communications (2016)
-
Relationship between left ventricular diastolic function and myocardial sympathetic denervation measured by 123I-meta-iodobenzylguanidine imaging in Anderson-Fabry disease
European Journal of Nuclear Medicine and Molecular Imaging (2016)