Abstract
New topochemistry in simple molecular systems can be explored at high pressure. Here we examine the binary nitrogen/hydrogen system using Raman spectroscopy, synchrotron X-ray diffraction, synchrotron infrared microspectroscopy and visual observation. We find a eutectic-type binary phase diagram with two stable high-pressure van der Waals compounds, which we identify as (N2)6(H2)7 and N2(H2)2. The former represents a new type of van der Waals host–guest compound in which hydrogen molecules are contained within channels in a nitrogen lattice. This compound shows evidence for a gradual, pressure-induced change in bonding from van der Waals to ionic interactions near 50 GPa, forming an amorphous dinitrogen network containing ionized ammonia in a room-temperature analogue of the Haber–Bosch process. Hydrazine is recovered on decompression. The nitrogen–hydrogen system demonstrates the potential for new pressure-driven chemistry in high-pressure structures and the promise of tailoring molecular interactions for materials synthesis.
Similar content being viewed by others
Introduction
The chemistry and equations of state of simple molecular systems (for example, N2, H2, H2O, CO2, CH4 and so on) in the condensed state are of great importance to planetary astrophysics, for the accurate characterization of energetic reaction products and for engineering new molecular compounds by means of pressure-induced chemistry. These are also model systems for understanding chemical bonding, testing fundamental condensed matter theory and probing potential quantum effects at high density.
Extensive studies have shown that pure N2 and pure H2 demonstrate subtle and complex high-pressure behaviour. In solid N2, quadrupole–quadrupole interactions give way to partially and later fully ordered states with increasing pressure, leading to a rich polymorphism at low-to-moderate pressures1. In contrast, structural changes in H2 are determined by the interplay between quantum effects and orientational ordering, keeping the molecular centers of mass on an hcp lattice over a very large pressure domain2. Recent work on N2 and H2 has focused particular attention on the role of structure in facilitating transitions to nonmolecular and metallic phases at ultra-high pressure. A polymeric form of N2 has been observed above 100 GPa and at high temperature, and may be an ideal high-energy density material if recoverable at ambient conditions3,4,5,6. In addition, metallic H2 is expected to demonstrate near room-temperature superconductivity and would represent the ultimate hydride, but has thus far eluded experimental efforts7,8.
Despite their fundamental nature and ubiquity, there has yet to be a comprehensive study of the full N2–H2 binary system, although previous studies have examined limited individual concentrations9,10. Concentration opens a new parameter space (along with pressure and temperature) for exploring new stoichiometric compounds, reactivity in the solid solution and potential paths for the synthesis and stabilization of phases with technological applications. This could lead to new H2-rich compounds for fuel cell technology11 or enable H2 metallization by confinement in a host lattice12.
The formation of van der Waals compounds containing N2 and H2 demonstrates the potential for new stoichiometries at modest pressures. The first such compounds were produced in combination with rare gases, Ar(H2)2 (ref. 13) and (N2)11He (ref. 14). H2 has since been shown to form a family of compounds, including (O2)3(H2)4 (ref. 15), CH4(H2)2 (ref. 16), Xe(H2)7 (ref. 17) and SiH4(H2)2 (ref. 18). There is obvious interest in manipulating pressure-driven chemistry to transform the weak van der Waals interactions into stronger bonds, increasing the likelihood of recovering high-pressure phases at ambient conditions.
Here, we examine the N2-H2 binary phase diagram at 300 K to investigate both the chemistry and stability of the high-pressure solid phases. We identify two new van der Waals compounds, (N2)6(H2)7 and N2(H2)2. We study their behaviour using a combination of single-crystal X-ray diffraction in the diamond anvil cell (DAC) and Raman and infrared (IR) spectroscopy to characterize the structural, vibrational and chemical properties. (N2)6(H2)7 presents an unexpected host–guest structure, in which an N2 lattice hosts clusters of H2 molecules. This compound shows evidence for a marked change from van der Waals to ionic interactions at 50 GPa and room temperature- a regime in which both end-member molecular species are otherwise quite stable. This unique pressure-induced change in bonding appears to be facilitated by the novel host–guest structure, overcoming the strong N2 triple bond at relatively modest conditions.
Results
Binary phase diagram
In this work, some 30 samples were prepared at 15 different concentrations to examine the full stoichiometric phase space as a function of pressure and to test thoroughly for reproducibility and chemical equilibrium in several regions of interest. Phase transitions were identified primarily by visual observation based on the difference in refractive index between phases, accompanied by Raman spectroscopy (described further below).
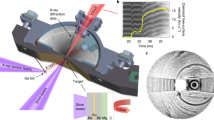
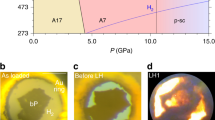
We identify a eutectic-type phase diagram with at least three triple points (near 35, 60 and 80 mol% H2 concentration) and two stoichiometric van der Waals compounds at 54 mol% and 66 mol% H2 concentration, respectively (Fig. 1). The liquidus line was determined by visual identification of the disappearance of single crystals and is represented by the locus of data points in Fig. 1a. The two species are completely miscible in the fluid phase, but have a low mutual solubility in the solid phase—<2 mol% N2 in solid H2 and <5 mol% H2 in solid N2. The shape of the liquidus, along with visual observation, confirms the existence of two stoichiometric compounds distinguished by their disparate hexagonal and cubic symmetry (Fig. 1b,c). Continuous pressure measurements during decompression to the fluid state at their precise stoichiometries confirm that they melt congruently.
(a) Fifteen concentrations were studied at room temperature. Two stoichiometric compounds exist at 54% and 66 mol% H2 concentration, respectively. Away from these concentrations, phase separation occurs between the van der Waals compounds and either a nitrogen-rich solid (S1) or a hydrogen-rich solid (S2). Data points represent visual observation of complete melting. S+F indicates solid–fluid equilibrium. The (N2)6(H2)7 compound has hexagonal symmetry, visual in a photomicrograph (b) of a single crystal in equilibrium with the fluid at 7 GPa. Similarly, single crystals of the N2(H2)2 solid are seen to be cubic as they grow in equilibrium with the fluid, (c). Black spheres in the lower right are ruby pressure gauges. Precession images (reconstructed from the raw X-ray diffraction data) show the (h0l) layer for both solids in (d) and (e). Because of the small mosaicity of the crystals and high detector resolution, the individual points are small and therefore emphasized with circles.
Structural study
We identify these compounds as (N2)6(H2)7 and N2(H2)2, respectively, on the basis of their H2 concentrations and single-crystal X-ray diffraction analyses. Samples were prepared as close to the ideal stoichiometries as possible such that the single-crystals could be grown to fill the sample chamber. In most cases, crystals were allowed to anneal for at least 24 h at room temperature and 1–2 GPa above the solidus before spectra were recorded. The single crystals are observed to have a small mosaicity in the X-ray data (0.05° in the detector plane), indicating that they are of high quality.
X-ray diffraction reveals that the N2(H2)2 compound (Fig. 1c) crystallizes in a cubic structure with the most probable space groups being Pm-3m and Pm-3n. This is reminiscent of the cubic structure of the δ-phase of N2 (Pm-3n) at similar pressures, but with a lattice parameter nearly twice as large (a=12.349 Å at 9.2 GPa, compared with 6.112 Å for δ-N2 at slightly lower pressure)19,20. A complete structural determination could not be achieved on the basis of the present data because of the rapid decrease of the reflection intensities with the diffraction angle, which we believe is due to orientational disorder of the molecules, as in δ-N2. Despite potential similarities with the case of Ar(H2)2 (ref. 13) and observations of AB2 Laves phases in other van der Waals compounds21, we do not believe that the present N2(H2)2 compound represents a Laves phase. Indeed, there is only one cubic Laves phase with the MgCu2-type structure (Fd3m) and the ratio of effective molecular radii does not match the criteria for optimum packing expected for such a phase. Instead, it appears more likely that H2 molecules either substitute in the N2 lattice or assume interstitial positions, leading to the distension of the unit cell in a δ-N2-like structure.
In contrast, the structure of the hexagonal solid, (N2)6(H2)7, differs entirely from the corresponding equilibrium structures in either N2 or H2 (Fig. 2). It is also unlike a previously reported N2–D2 structure9, which was not apparent in our experiments. Instead, we identify a novel host–guest compound with 36 N2 and 42 H2 molecules in a rhombohedral unit cell of R-3m space group. The lattice parameters for the conventional triple hexagonal cell are a=b=14.273(3) Å, and c=8.075(9) Å at 8 GPa. Full crystallographic details are given in Supplementary Table 1. As illustrated in Fig. 2, a cage-like lattice of N2 molecules confines clusters of rotationally disordered H2 molecules. Helical chains of N2 molecules (emphasized in red, Fig. 2b) extend along the <001> direction and confine intermediate N2 molecules in a puckered hexagonal configuration (emphasized in green). These effectively form the ends of large, open polyhedral volumes (Fig. 2c), each of which hosts 15 H2 molecules (two of which are shared with the neighbouring volumes, above and below, forming channels along the c-axis). This host–guest configuration is radically different from most van der Waals compounds, which often assume structures according to ideal hard-sphere packing configurations (for example, Ar(H2)2, CH4(H2)2, Xe(O2)2)13,16,21 or share a close structural resemblance with one of the constituents (for example, (N2)11He) (ref. 22). It is also notable that although N2 molecules maintain rotational degrees of freedom in the pure phase (δ-N2), here they are oriented. The presence of the guest H2 molecules and the resulting quadrupolar interactions in the novel structure favour the formation of an ordered lattice, despite the average intermolecular distances being slightly greater than in pure N2. Cage-like structures, such as this, may represent an efficient means of ‘chemical compression’ by enhancing confinement of the guest molecules. Because of the potential for unique interactions in this novel host–guest structure, we set out to explore the physical and chemical behaviour of (N2)6(H2)7.
(a) Single-crystal X-ray diffraction reveals that clusters of H2 molecules (shown in grey) are confined in channels within an N2 lattice (blue dumbbells). (b) Helical chains of N2 (emphasized in red) orient intermediate N2 molecules in a puckered, hexagonal arrangement (emphasized in green) roughly in the <001> plane. (c) This generates a channel comprised of cage-like volumes along the c-axis, each of which hosts 15 H2 molecules. Two of these are shared with the neighbouring volumes. Complete crystallographic data are included in Supplementary Table 1.
Raman and IR spectroscopy reveal pressure-induced chemistry
Experiments were carried out on compression and decompression to 60 GPa using Raman and IR spectroscopy to investigate changes in bonding in the (N2)6(H2)7 compound. Separate experiments were carried out to 160 GPa to track changes in IR and visible absorption. The pressure evolution of the vibron modes is shown in Fig. 3. H2 exhibits three IR-active vibron modes and three Raman-active modes. The highest frequency branch agrees closely in frequency and is likely shared (both Raman- and IR-active), whereas the lower frequency branches may be ascribed to different modes. All observed modes are blue-shifted with respect to pure H2 due to partial suppression of the vibrational coupling, as observed in other H2/rare gas compounds23,24,25. In addition, the H2 rotational mode near 600 cm−1 is seen to persist to 50 GPa, confirming that hydrogen remains rotationally free, in contrast to its N2 host lattice (Supplementary Fig. 5). Two Raman active modes and two IR active modes are observed for N2, with vibrational frequencies that closely follow the ν2 mode of pure nitrogen and its branching with pressure. It is notable that IR activity begins for both species on crystallization. This is in stark contrast to the pure phases, where the IR modes appear at 20 and 215 GPa for N2 and H2, respectively. This indicates an induced dipolar moment with increasing pressure, not typically present in a homonuclear diatomic molecule (Fig. 3c).
(a) Evolution of Raman and infrared vibrons for the (N2)6(H2)7 inclusion compound (54 mol% H2). Red dashed curves are from refs 25, 45. (b) Visual Raman data taken on compression show the appearance of a new, broad feature at 2,370 cm−1, which appears near 47 GPa and grows at the expense of the υ2 mode of N2 with increasing pressure (indicated by an arrow). Following the appearance of this mode, the N2 and H2 vibrons diminish and then disappear with time (as shown at 58 GPa), suggesting a (sluggish) transition to an amorphous phase in which the previously inert molecules have reacted. (c) Infrared absorption data, also on compression, were collected on a separate sample. IR vibrons also disappear with time and with increasing pressure as a new, high-pressure phase forms from the previously crystalline solid.
At 49 GPa, a marked change in vibron signature is observed. The H2 symmetric stretching modes disappear, whereas the N2 Raman modes give way to a broad, lower-frequency feature (Fig. 3b), suggesting a possible amorphization. The amorphous state was later confirmed by the absence of X-ray diffraction peaks above the transition pressure. Despite this significant change, the solid appears to remain macroscopically intact with no visible phase separation. The transition occurs sluggishly throughout the sample, going to completion in a matter of hours and can be overdriven by as much as 15 GPa under very rapid compression. In time, however, a very broad feature appears in the Raman data in regions associated with N–H symmetric and antisymmetric stretch modes (~2,900–3,600), suggesting disruption of the strong N2 triple bond and the onset of a chemical transformation in the amorphous, reacted solid (Fig. 4).
(a) Following the pressure-induced chemical reaction in (N2)6(H2)7 near 50 GPa, a broad band is observed in infrared absorption spectra above 2,000 cm−1 (shaded), suggesting the presence of NH4+/NH2− in the high-pressure phase and the breakdown of van der Waals interactions in the solid. With decreasing pressure, N-H stretching modes become increasingly resolved and resemble those of NH3 (green)28, suggesting reaction of the species. (b) Visible Raman data demonstrate extreme broadening and slight redshift of the principle N2 stretch mode (2,300–2,400 cm−1) in the high-pressure phase, suggesting an amorphous state. Broad N–H stretch modes (already evident at high pressure, 3,000–3,500 cm−1) become increasingly structured on decompression and are consistent with the recovery of a mixture of pure N2 and hydrazine (N2H4)29,46. The peak at ~1,100 cm−1 is consistent with an N–N stretch mode (indicated by a *), whereas the others (indicated by grey arrows) may be assigned to N–H bending, stretching and deformational modes.
To better understand this transition, we probed the forward and reverse transformations in several loadings, collecting IR, Raman and visible-absorption measurements. Typical IR and Raman spectra obtained on decompression of the amorphous solid are shown in Fig. 4a,b, and suggest that the high-pressure phase has an ionic character. Most significantly, we note that IR absorption is enhanced with a broad absorption feature between ~2,100–2,900 cm−1 (shaded region, Fig. 4a). These spectra bear a strong resemblance to recent experiments and theory on the ammonium amide phase (NH4+/NH2−), shown to result from the auto-ionization of NH3, albeit at much more extreme conditions26,27,28. The broadband centered near 1,500 cm−1 is consistent with the bending modes of NH4+ and NH2−. With increasing pressure, a strong visible absorption band is also observed between ~400–500 nm, resulting in a darkening of the sample (Supplementary Fig. 6). Together with the IR data, this suggests the onset of increased charge transfer between nitrogen and hydrogen, signalling the formation of ionized NH3 in an amorphous N2 structure.
On decompression, the visible absorption band blueshifts and decreases in intensity, accompanied by distinct structuring of N–H Raman and IR vibrational modes. The ionic character of the sample appears diminished below ~40 GPa. The breadth and relative intensity of the Raman modes are potentially consistent with an NH3-rich solid at higher pressures (Fig. 4b), however with decreasing pressure the stretching modes shift to lower frequency and become increasingly structured, evolving towards the spectral signature of N2H4 hydrazine)29,30. Below 9 GPa, the N2 vibron band progressively transforms into two well-resolved peaks close to those of pure N2. The solid only slightly deforms before a clear phase separation between liquid N2 and solid N2H4, well below the melting pressure of the original (N2)6(H2)7 compound (Supplementary Fig. 7) confirming that a reaction has taken place. Excess H2 may be present but was not detected.
Discussion
The structure of the (N2)6(H2)7 compound appears to facilitate intermolecular interactions sufficient to break down the strong N≡N triple bond and promote N–H bonding above 50 GPa, resulting in chemical behaviour reminiscent of that seen in other nitrogen compounds at much more extreme conditions. Indeed, the auto-ionization of NH3 to form ammonium amide (NH4+/NH2−) was seen at pressures more than twice as high as our observations27,28. Mixtures of nitrogen and oxygen have also been shown to transform from molecular compounds to ionic solids (nitrosonium nitrate), though only at high temperatures31 or when subjected to laser or X-ray irradiation32. Similarly, pure N2 exhibits an amorphous, narrow band-gap, nonmolecular phase (η) but only at pressures above 150 GPa1,5,33,34,35. The present results demonstrate the possibility of manipulating the topochemistry of simple mixtures to markedly reduce the thresholds for pressure-induced reactions, leading to behaviour otherwise observed at much higher pressures and/or temperatures. Such high-pressure phases have tremendous potential for inducing transitions from predominantly intra- to intermolecular interactions, well within the stability regimes of the end-member constituents, as demonstrated by the observed shift from van der Waals to ionic interactions in (N2)6(H2)7. This is a promising avenue for exploring ‘tunable’ pressure-induced chemistry and potentially lowering activation barriers for favourable reactions in other molecular systems.
We have presented a preliminary nitrogen–hydrogen binary phase diagram, which shows evidence for two new van der Waals compounds. (N2)6(H2)7 was characterized and shown to undergo a change from van der Waals to ionic interactions coincident with a transformation to an amorphous phase near 50 GPa. On decompression, spectroscopic data show that N–H bonds evolve in the reacted solid, forming ammonium amide at high pressures and then leading to the formation of N2H4 in equilibrium with liquid N2 on melting. These results demonstrate that very interesting molecular arrangements such as the remarkable host–guest structure of (N2)6(H2)7 can be obtained through the pressure-driven formation of van der Waals compounds. Additional stimuli (for example, photochemistry by irradiation) may further facilitate such reactions and if similar structures can be stabilized and recovered, they may represent a means of engineering solids with desirable technological applications. Other such structures may also present unique opportunities for the chemical confinement of molecular H2. The stability and properties of (N2)6(H2)7 at low temperature will be the subject of future studies, and we hope that this work will motivate experimental and theoretical structural searches in other binary mixtures.
Methods
Sample preparation
Nitrogen–hydrogen mixtures were prepared in membrane DACs at either 20 MPa from standard bottles or 140 MPa using a gas-loading compressor. Partial gas pressures for a given concentration were calculated using a second-order virial correction to account for interaction of mixing and mixtures were left to equilibrate for at least 24 h before loading. Uncertainties in concentration are <1 mol %. Low-pressure experiments (<30 GPa) used CuBe gaskets and Boehler-Almax-type bevelled anvils with 300 μm culets. High pressures (>30 GPa) were achieved using Au-lined rhenium gaskets and diamond culets ranging from 40 to 100 μm with the sample diameter being ~1/3–1/2 that of the culet in all cases. Single crystal samples were grown under quasi-hydrostatic conditions from solid-fluid equilibrium by pressurization at room temperature and pressures were determined using the ruby fluorescence scale and/or the frequency shift of the first-order diamond phonon36,37,38.
Spectroscopy measurements
Visible Raman spectroscopy was performed in backscattering geometry and the 487.986 nm line from a continuous Ar laser, focused to ~3 μm at the sample. Data were recorded using a gain-amplified charge-coupled device (CCD) coupled to an Andor spectrometer, resulting in a spectral resolution of ~2 cm−1. Synchrotron IR spectroscopy was carried out on the SMIS beamline at the Soleil synchrotron between 650 and 8,000 cm−1 using a coupled Fourier Transform Infrared (FTIR) spectrometer39. Additional IR measurements were conducted using a custom benchtop microspectroscopy system40. The empty DAC was used as a reference for treatment of the absorption data.
X-ray diffraction and structural analysis
Structural information was obtained from angle-dispersive single-crystal X-ray diffraction carried out on beamline ID09 (ref. 41) of the European Synchrotron Radiation Facility (Grenoble, France), using a focused (~20 μm) monochromatic beam at λ=0.4147 Å (29.8 keV). Data were recorded on a MAR 555 flat-panel detector calibrated with a Si standard. The sample was rotated in 0.5° oscillations over a range of 58° about the vertical axis during collection. CrysalisPro (Oxford Diffraction) was used to analyze the images and index reflections. The structure of the (N2)6(H2)7 compound was solved using the ab initio charge-flipping algorithm, Superflip42. SHELXL43 and Olex2 (ref. 44) software packages were used for structural refinement. Complete details of the structural analysis are provided in the Supplementary Discussion and Supplementary Data 1. These techniques, together with visual microscopic observation, provide a complete suite of data on the vibrational, structural and macroscopic characteristics of the sample.
Additional information
How to cite this article: Spaulding, D. K. et al. Pressure-induced chemistry in a nitrogen-hydrogen host–guest structure. Nat. Commun. 5:5739 doi: 10.1038/ncomms6739 (2014).
References
Gregoryanz, E. et al. High P-T transformations of nitrogen to 170GPa. J. Chem. Phys. 126, 184505 (2007).
McMahon, J., Morales, M., Pierleoni, C. & Ceperley, D. M. The properties of hydrogen and helium under extreme conditions. Rev. Mod. Phys. 84, 1607–1653 (2012).
Eremets, M., Gavriliuk, A. G., Trojan, I. A., Dzivenko, D. A. & Boehler, R. Single-bonded cubic form of nitrogen. Nat. Mater. 3, 558–563 (2004).
Ciezak, J. in Metastable Polymeric Nitrogen: the Ultimate Green High-Energy Density Material. The U.S. Army Research Laboratory Report ARL-TR-4478 (2008).
Lipp, M. J. et al. Transformation of molecular nitrogen to nonmolecular phases at megabar pressures by direct laser heating. Phys. Rev. B 76, 014113 (2007).
Zahariev, F., Dudiy, S. V., Hooper, J., Zhang, F. & Woo, T. K. Systematic method to new phases of polymeric nitrogen under high pressure. Phys. Rev. Lett. 97, 155503 (2006).
Loubeyre, P., Occelli, F. & Le Toullec, R. Optical studies of solid hydrogen to 320GPa and evidence for black hydrogen. Nature 416, 613–617 (2002).
Zha, C., Liu, Z., Ahart, M., Boehler, R. & Hemley, R. J. High-pressure measurements of hydrogen phase IV using synchrotron infrared spectroscopy. Phys Rev. Lett. 110, 217402 (2013).
Kim, M. & Yoo, C.-S. Highly repulsive interaction in novel inclusion D2-N2 compound at high pressure: Raman and X-ray evidence. J. Chem. Phys. 134, 044519 (2011).
Ciezak, J., Jenkins, T. A. & Hemley, R. J. inProceedings Shock Compression of Condensed Matter eds. Elert M. L., Butler W. T., Anderson W. W., Proud W. G. 1291–1294American Institute of Physics (2009).
Jena, P. Materials for hydrogen storage: past, present, and future. J. Phys. Chem. Lett 2, 206–211 (2011).
Ashcroft, N. W. Hydrogen dominant metallic alloys: high temperature superconductors? Phys Rev. Lett. 92, 187002 (2004).
Loubeyre, P., LeToullec, R. & Pinceaux, J. P. Compression of Ar(H2)2 up to 175GPa: a new path for the dissociation of molecular hydrogen? Phys. Rev. Lett. 72, 1360–1363 (1994).
Vos, W. L. & Schouten, J. A. The stability of van der Waals compounds at high pressures. Fiz. Nizk. Temp. (Low Temp. Phys.) 19, 481–485 (1993).
Loubeyre, P. & Le Toullec, R. Stability of O2/H2 mixtures at high pressure. Nature 378, 44–46 (1995).
Somayazulu, M., Finger, L. W., Hemley, R. J. & Mao, H. K. High-pressure compounds in methane-hydrogen mixtures. Science 271, 1400–1402 (1996).
Somayazulu, M. et al. Pressure-induced bonding and compound formation in xenon-hydrogen solids. Nat. Chem. 2, 50–53 (2009).
Strobel, T., Somayazulu, M. & Hemley, R. J. Novel pressure-induced interactions in silane-hydrogen. Phys. Rev. Lett. 103, 065701 (2009).
Cromer, D. T., Mills, R. L., Schiferl, D. & Schwalbe, L. A. The structure of N2 at 49kbar and 299K. Acta Crystallogr. B37, 8–11 (1981).
Stinton, G. W., Loa, I., Lundegaard, L. F. & McMahon, M. The crystal structures of delta and delta* nitrogen. J. Chem. Phys. 131, 104511 (2009).
Weck, G., Dewaele, A. & Loubeyre, P. Oxygen/noble gas binary phase diagrams at 296K and high pressures. Phys. Rev. B 82, 014112 (2010).
Ninet, S., Weck, G., Loubeyre, P. & Datchi, F. Structural and vibrational properties of the van der Waals compound (N2)11He up to 135GPa. Phys. Rev. B. 83, 134107 (2011).
Loubeyre, P., Le Toullec, R. & Pinceaux, J. P. Properties of H2 under strong compression in a Ne matrix. Phys. Rev. Lett. 67, 3271–3274 (1991).
Loubeyre, P., Toullec, R. & Pinceaux, J. P. Raman measurements of the vibrational properties of H2 as a guest molecule in dense helium, neon, argon, and deuterium systems up to 40GPa. Phys. Rev. B 45, 12844–12853 (1992).
Occelli, F. Physique de l’Hydrogene a Haute Pression, PhD thesis, Univ. Paris VI (2002).
Pickard, C. J. & Needs, R. J. Highly compressed ammonia forms an ionic crystal. Nat. Mater. 7, 775–779 (2008).
Palasyuk, T. et al. Ammonia as a case study for the spontaneous ionization of a simple hydrogen-bonded compound. Nat. Commun 5, 3460 (2014).
Ninet, S. et al. Experimental and theoretical evidence for an ionic crystal of ammonia at high pressure. Phys. Rev. B 89, 174103 (2014).
Jiang, S. et al. Hydrogen Bond in Compressed Solid Hydrazine. J. Phys Chem. C 118, 3236–3243 (2014).
Ninet, S., Datchi, F., Saitta, A. M., Lazzeri, M. & Canny, B. Raman Spectrum of Ammonia IV. Phys. Rev. B 74, 104101 (2006).
Somayazulu, M. et al. Novel broken symmetry phase from N2O at high pressures and high temperatures. Phys. Rev. Lett. 87, 135504 (2001).
Sihachakr, D. & Loubeyre, P. High-pressure transformation of N2/O2 mixtures into ionic compounds. Phys. Rev. B 74, 064113 (2006).
Gregoryanz, E., Goncharov, A. F., Hemley, R. J. & Mao, H. K. High-pressure amorphous nitrogen. Phys. Rev. B 64, 502103 (2001).
Gregoryanz, E. et al. Raman, infrared, and X-ray evidence for new phases of nitrogen at high pressures and temperatures. Phys. Rev. B 66, 224108 (2002).
Goncharov, A. F., Gregoryanz, E., Mao, H. K., Liu, Z. & Hemley, R. J. Optical evidence for a nonmolecular phase of nitrogen above 150GPa. Phys. Rev. Lett. 85, 1262–1265 (2000).
Piermarini, G. J., Block, S., Barnett, J. D. & Forman, R. A. Calibration of the pressure dependence of the R1 ruby fluorescence line to 195kbar. J. Appl. Phys. 46, 2774–2780 (1975).
Datchi, F. et al. Optical pressure sensors for high-pressure-high-temperature studies in a diamond anvil cell. High Press. Res 27, 447–483 (2007).
Akahama, Y. & Kawamura, H. High-pressure Raman spectroscopy of diamond anvils to 250GPa: Method for pressure determination in the multimegabar pressure range. J. Appl. Phys. 96, 3748–3751 (2004).
Dumas, P. et al. Synchrotron Infrared Microscopy at the French Synchrotron facility SOLEIL. Infrared Phys. Techn. 49, 152–160 (2006).
Andre, R. inMicro Spectroscopie Raman Par Transformée de Fourier Proche Infrarouge Editions Universitaires Europeennes (2011).
Merlini, M. & Hanfland, H. Single-crystal diffraction at megabar conditions by synchrotron radiation. High Press. Res. 33, 511–522 (2013).
Palatinus, L. & Chapuis, G. SUPERFLIP – a computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Cryst. 40, 786–790 (2007).
Sheldrick, G. A short history of SHELX. Acta Cryst. A 64, 112–122 (2008).
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst. 42, 339–341 (2009).
Schneider, H., Hafner, W., Wokaun, A. & Olijnyk, H. Room temperature Raman scattering studies of external and internal modes of solid nitrogen at pressures 8<P<54GPa. J. Chem. Phys. 96, 8046–8053 (1992).
Chellappa, R., Dattelbaum, D., Daemen, L. & Liu, Z. High pressure spectroscopic studies of hydrazine (N2H2), Proceedings 18th APS-SCCM and 24th AIRAPT, J. Phys. Conf. Series 500, 052008, IOP Publishing (2014).
Acknowledgements
We thank Mr Ramesh André and Dr Florent Ocelli for their assistance with sample preparation, experiments and helpful discussions.
Author information
Authors and Affiliations
Contributions
D.K.S., G.W. and P.L. designed the project. D.K.S. and G.W. conducted the experiments and analyzed the data. F.D. assisted with structural analyses and interpretation. P.D. and M.H. assisted with synchrotron experiments. D.K.S., G.W., P.L. and F.D. wrote the manuscript. All authors discussed results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures, Supplementary Tables, Supplementary Discussion and Supplementary References.
Supplementary Figures 1-8, Supplementary Table 1, Supplementary Discussion and Supplementary References (PDF 6271 kb)
Supplementary Data 1
The supplementary dataset contains the full output reports from the Crysalis Pro software used for analysis of the x-ray diffraction data. (PDF 250 kb)
Rights and permissions
About this article
Cite this article
Spaulding, D., Weck, G., Loubeyre, P. et al. Pressure-induced chemistry in a nitrogen-hydrogen host–guest structure. Nat Commun 5, 5739 (2014). https://doi.org/10.1038/ncomms6739
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms6739
This article is cited by
-
Aromatic hexazine [N6]4− anion featured in the complex structure of the high-pressure potassium nitrogen compound K9N56
Nature Chemistry (2023)
-
Materials discovery at high pressures
Nature Reviews Materials (2017)
-
Hexacoordinated nitrogen(V) stabilized by high pressure
Scientific Reports (2016)
-
Nitrogen Backbone Oligomers
Scientific Reports (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.