Abstract
Cooperation fundamentally contributes to the success of life on earth, but its persistence in diverse communities remains a riddle, as selfish phenotypes rapidly evolve and may spread until disrupting cooperation. Here we investigate how evolutionary history affects the emergence and spread of defectors in multispecies communities. We set up bacterial communities of varying diversity and phylogenetic relatedness and measure investment into cooperation (proteolytic activity) and their vulnerability to invasion by defectors. We show that evolutionary relationships predict the stability of cooperation: phylogenetically diverse communities are rapidly invaded by spontaneous signal-blind mutants (ignoring signals regulating cooperation), while cooperation is stable in closely related ones. Maintenance of cooperation is controlled by antagonism against defectors: cooperators inhibit phylogenetically related defectors, but not distant ones. This kin-dependent inhibition links phylogenetic diversity and evolutionary dynamics and thus provides a robust mechanistic predictor for the persistence of cooperation in natural communities.
Similar content being viewed by others
Introduction
Many unicellular organisms show complex social behaviours in which individuals actively communicate and cooperate1. Several bacteria secrete diffusible compounds that function as public goods benefiting all present organisms independently of their investment. Public goods include iron-scavenging siderophores2, extracellular enzymes3 or secondary metabolites harming competitors and predators4. Such cooperative traits improve growth rate, competitiveness and virulence of pathogens. However, if the cooperative traits are costly, they may also be vulnerable to exploitation by cheats reaping benefits without sharing the costs5. Defectors have been reported to win over cooperators over a broad range of frequencies, calling for mechanisms explaining the persistence of cooperation6. Many bacteria use quorum sensing (QS) signalling to adjust the investment into public goods to the potential benefits7,8. Investment in QS-regulated traits typically increases with population density8, allowing to maximize benefits-to-costs ratio by investing in social behaviour in the presence of cooperating neighbours. This increased investment, however, is susceptible to cheating. In bacteria, defectors may emerge by evolution of signal blindness9, where an individual stops responding to QS signals10. Defectors’ fitness typically depends on the investment of cooperators into public goods11. Rare defectors may therefore show a high fitness12 and invade if cooperators do not selectively enforce cooperation by harming defectors13.
The establishment and stability of cooperation have been extensively studied using single pairs of cooperators and defectors, predicting defector’s fitness as a function of cooperators investment in public goods and spiteful behaviours14. Most studies use the Hamiltonian kin selection framework15 predicting that altruism becomes evolutionary stable as relatedness augments. In a multispecies system, this concept can be extended to evolutionary history: because of their common ancestry, different species may share compatible kin recognition mechanisms and perceive each other as partner, although differing at the level of the cooperative locus16,17.
Bacteria live in complex communities forming networks of antagonistic or facilitative interactions18,19, with very little being known on the dynamics of cooperative behaviours in multispecies microbial communities. Diversity does not impede cooperation per se20, but makes it difficult to predict using the Hamiltonian framework. In multispecies communities, cross-talk between unrelated species may result in cooperative behaviour17. Further, antagonistic interactions, as caused by the production of bacteriocins or antibiotics, may harm a broad range of competitors21, independent of their cooperativeness or shared evolutionary history with the emitter strain22.
We investigated how biodiversity affects the stability of cooperation. We set up mixed communities ranging from one to eight Pseudomonas spp. genotypes, a model taxon in ecological and evolutionary research3,23. The communities varied in two diversity metrics, the richness (number of genotypes) and evolutionary history (mean pairwise phylogenetic distance), two widely used indices with contrasting effects on community performance24. We grow the bacteria in quorum sensing medium (QSM)3, a minimal medium with albumin as the sole carbon source. Albumin first has to be degraded by extracellular proteases, which function as a QS-regulated public good in Pseudomonas25. Fluorescent pseudomonads produce extracellular proteases under the control of the gac–rsm QS system and may respond to their own as well as other species’ QS signals17,25. We monitor the apparition of spontaneous protease-deficient defectors in communities of varying phylogenetic history and richness. We expect that if high genetic relatedness between strains promotes their ability to cooperate, community phylogenetic history will predict the fate of cooperation. On the other hand, if non-additive diversity effects drive the fitness of defectors24, we expect that community richness would be the best predictor of the stability of cooperation. We further set up a second experiment with a focal-defined defector and followed its fitness as a function of its original frequency, community richness and mean phylogenetic distance between the defector and surrounding cooperators. We then analyse if biodiversity effects on defector success can be attributed to the investment into public goods and defector inhibition by the cooperating genotypes.
We observe that the stability of cooperation is directly proportional to the mean phylogenetic relatedness between the present genotypes. Communities containing phylogenetically close species keep cooperating, whereas communities containing phylogenetically distant species get rapidly invaded by defectors emerging by spontaneous mutation. We show that this pattern is due to antagonistic interactions, as cooperators can inhibit defectors from closely related genotypes but not from unrelated ones.
Results
Spread of evolved defectors
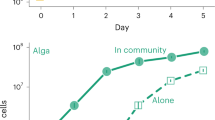
In this first experiment, multispecies communities of cooperators are growing on albumin as the sole carbon source. Bacteria must produce proteases to break down and assimilate the nutrients. In pseudomonads, protease-deficient mutants rapidly emerge by mutation of the gacS/gacA QS cascade26. In our experiment, protease-deficient phenotypes rapidly evolve out of initially cooperating communities. Their spread is further closely correlated to the mean pairwise phylogenetic distance (generalized linear model (GLM), F1,46=112.1, P<0.001; Fig. 1), but not to the richness (GLM, F1,46=0.8, P=0.36 when fitted sequentially after mean pairwise phylogenetic distance) of the community: although communities of phylogenetically related bacteria remain virtually free of defectors, phylogenetically diverse communities rapidly accumulate protease-deficient defectors. None of these defector isolates could grow on QSM alone, even with addition of signals from the cooperators, suggesting that these were signal-blind defectors relying on the public goods (proteases) secreted by the cooperators. The phenotype of the isolated defectors is typical for mutations in the gacS/gacA QS cascade27, a rapidly mutating system whose deactivation results in signal blindness and accounts for virtually all protease-deficient mutants in Pseudomonas spp.9,28.
Communities (100% cooperators at the beginning of the experiment) were grown on QSM medium for 48 h. Signal-blind defectors were discriminated by their lack of proteolytic and tryptophan dioxygenase activity and expressed as per cent of the community after 48 h (GLM, R2=0.72, P<0.001). Symbol shading shows the richness of the different communities: white, monocultures; light grey, two genotypes; dark grey, four genotypes; and black, eight genotypes.
As defector fitness may be related to the cooperators’ investment into public goods11, we further test if the defector load is correlated with the proteolytic activity of the community. Under the tested conditions, proteolytic activity is unaffected by the genetic distance between the genotypes present (GLM, R2=0.01, P=0.90), suggesting that phylogenetically distant genotypes engage into cooperative behaviours. Proteolytic activity only partially predicts defector load (GLM, R2=0.10, P=0.04), suggesting that other interactions such as antagonistic ones may drive the fitness of emerging defectors.
Phylogenetic constraints on defector inhibition
To uncover antagonistic interactions, we investigate if cooperators selectively harm defectors and if harming is affected by phylogenetic distance. In bacteria, inhibition of competitors often relies on diffusible toxins29, prompting us to test if cooperators secrete metabolites harming defectors. We use an isogenic signal-blind mutant of one of the used genotypes (Pseudomonas fluorescens CHA0ΔgacS30) as model defector of defined genetic background. This mutant is typical for spontaneous mutations deactivating secondary metabolism in pseudomonads28 and lacks extracellular proteases required for growing on albumin3,30. It grows better than the wild type when cooperation is not required4, but preliminary experiments showed that it is incapable of growing on QSM medium alone. In order to investigate its growth inhibition by the cooperators, we grow this mutant under non-limiting nutrient conditions (Lysogeny Broth (LB)) supplemented with small amounts of supernatant from communities grown in QSM. Supernatants inhibit the growth of the signal-blind mutant and the inhibition is negatively correlated with the mean phylogenetic distance between the community and the defector (GLM, R2=0.44, P<0.001; Fig. 2), but not to community richness (GLM, R2=0.08, P=0.4). To exclude effects due to the genetic background, we also assess the response of the wild-type CHA0, with no significant effect of phylogenetic distance on its growth (GLM, R2=0.01, P=0.9). These results suggest that cooperators produce diffusible toxins specifically suppressing phylogenetically close defectors.
Effect of mean genetic distance between the defector and the community of cooperating bacteria on the growth inhibition of the model defector Pseudomonas fluorescens CHA0ΔgacS by cell-free supernatant of a 48-h-old cooperator cultures varying in mean genetic distance to the defector (GLM, R2=0.44, P<0.001). Inhibition was expressed as the growth reduction compared with control, normalized by the growth reduction of the wild-type background genotype CHA0. Note that to improve the robustness of the prediction, 96 communities were tested instead of the 48 used in the competition experiment. Symbol shading shows the richness of the different communities, ranging from white (monocultures) to black (eight genotypes).
Frequency-dependent defector fitness
To validate if defector inhibition rather than investment into public goods may explain the low defector load of phylogenetically related communities, we model and test experimentally frequency-dependent fitness of defectors under various cooperation and harming scenarios. We simulate defector payoff at different initial frequencies with a modified snowdrift model31. This game theory model considers payoff of defection as a function of the investment of the whole community into public goods. We complete this model by adding a defector inhibition function based on fully diffusible toxins. Assuming that QS regulates the production of both public goods and toxins25, the per capita and population-level investment in these two traits will increase as a linear32 and quadratic function of cooperator frequency, respectively. The model predicts that payoff (benefits minus costs) of defection will be negatively frequency dependent in the absence of specific inhibition of the defectors (Fig. 3a), and reflects well previous studies on the negative frequency-dependent fitness of defectors12. As this inhibition increases, payoff becomes hump-shaped, resulting in the defector to go extinct except at intermediate frequency. As the toxin function peaks at low defector frequency, low toxicity of the cooperators towards the defectors is sufficient to annihilate the advantage of rare defectors, stabilizing cooperation by preventing emerging defectors from invading (Fig. 3a).
(a) Simulation of defector payoff as a function of defector initial frequency and presence of compounds specifically inhibiting defectors. A positive relative payoff (blue region) indicates higher fitness of the defector, whereas a negative one indicates higher fitness of the cooperator (red region). (b) Empirical data showing the fitness of the model defector Pseudomonas fluorescens CHA0ΔgacS as a function of its initial frequency and its mean genetic distance to the community of cooperating bacteria. A fitness value >1 (blue region) indicates faster growth of the defector, whereas a value between 0 and 1 (red region) indicates faster growth of the cooperator. The isoclines correspond to the polynomial surface regression reported in Table 1 (R2=0.56, P<0.0001). Grey circles represent the individual communities.
We validate the model by growing the signal-blind CHA0ΔgacS at various initial frequencies in QSM with the bacterial communities described above. Defector fitness matches the predictions from the model: in the presence of phylogenetically distant (and thus inefficiently inhibiting defectors) cooperator communities defector fitness is negatively frequency-dependent (Fig. 3b). This corresponds well to predictions of defector fitness without antagonistic interactions12. Fitness is particularly high at low frequency, where small numbers of defectors can exploit large amounts of public goods, so that evolved defectors can rapidly invade. In contrast, the fitness of the mutant facing phylogenetically close (and efficiently inhibiting defectors) cooperators is hump-shaped and peaks at intermediate frequency with rare mutants having very low fitness and being unable to invade (Fig. 3b). Mutant fitness can be well predicted as a function of its initial frequency and mean phylogenetic distance to the remaining community (Table 1). Defector inhibition is the best predictor of mutant fitness (initial frequency × defector inhibition interaction; Table 1), surpassing the effect of public good production (proteolytic activity).
Discussion
Biodiversity is an important driver of ecological processes. In this study, we show that evolutionary history also predicts the stability of cooperation in multispecies communities (Fig. 4). Interestingly, investment in public goods (proteases) was independent of the phylogenetic distance within the community. The gac/rsm QS pathway is highly conserved among Pseudomonas spp.25, and phylogenetically distant species engage in cooperation due to an overlap in QS signals17. Using ’leaky’ communication systems open to cross-talk may be a powerful way of optimizing cooperation as resource differentiation between competitors positively correlates with phylogenetic distance24. In an ideal scenario, distant species cooperate making a common resource available. Each species then uses different resource fractions thereby reducing competition. In this scenario, a phylogenetically closely related defector is more threatening than an unrelated cooperator, as it is not contributing to opening up resources but resource consumption may highly overlap with the cooperator. In agreement with this conflict, defector inhibition decreased with increasing phylogenetic distance. Cooperators rapidly evolve mechanisms that prevent defection33, and in our experiment the ability of cooperators to constrain the emergence of defectors is closely linked to their genetic relatedness. Owing to the high rate of QS disrupting mutations9,27 and the fact that the emerging mutants are imbedded in populations of the cooperating parental strain, the necessity of the parental strain to control the spread of defecting clone mates likely exceeds that of controlling invasion by unrelated defectors. Invasion by unrelated defectors occurs from outside the population, whereas signal-blind defector mutants emerge by cell division inside the population of the cooperating ancestor. Based on our results, we propose that inhibition of closely related defectors was due to a combination of policing, in which cooperators invest into harming of defectors34, and disruption of QS via pleiotropic effect reducing resistance to various biotic and abiotic stressors4,35. The increased sensitivity of defectors to genetically related cooperators points to a suppression of close kin refusing to cooperate. This resembles policing in social Hymenoptera suppressing reproductive workers that reinforces cooperation once established36; however, in social Hymenoptera policing increases with genetic distance37 whereas in P. fluorescens it is most pronounced against closely related defectors. As a result, mixtures of closely related genotypes will stably cooperate because the emerging defector suffers from toxins of all the clonal and close kin cells of the parental strain. Mixtures of more distant genotypes will still cooperate, but will not be able to control the emergence of defectors.
We observe that defector inhibition is the main predictor of defector spread. As phylogenetic distance between competing species increase, the investment into cooperation remains stable, but the efficiency of defector inhibition decreases. Based on the mathematical prediction and the experimental data, this reduced inhibition favours the spread of defectors: in closely related communities (a), cooperators not only produce public goods but also toxins efficiently inhibiting defectors. Rare defectors (for example, emerging by mutation) face high toxicity cancelling the advantage of defection and cannot invade communities of cooperators. In contrast, in loosely related communities (b), rare defector profit from the high investment into public goods by the cooperators. Defector fitness is negatively frequency dependent, and rare defectors may invade. Functions for both public goods and defector inhibition are quadratic, reflecting that per capita investment by cooperators is density-dependent (quorum-sensing-regulated).
Overall, the results suggest that uncoupling of phylogeny and cooperation helps in explaining one of the major riddles in evolutionary biology: the stability of cooperation. Cooperation is facilitated by kin selection as genetically related organisms share genes. However, related organisms also occupy similar niches and use similar resources, which increases competition38. Evolutionary history has been shown to drive the productivity of plant and microbial communities24,39, and we show that it may also affect the evolution of new phenotypes. Suppression of genetically related defectors was the major determinant of the stability of cooperation in multispecies microbial communities. Increasing phylogenetic distance between the species of a community did not directly decrease investment into cooperation, but rather the ability of the cooperators to control the spread of defectors. The number of genotypes present did not affect the relationship between phylogenetic distance and defector fitness, suggesting that this study may serve to predict the fate of cooperation in more complex communities. Therefore, the present study links phylogenetic structure to evolutionary dynamics of bacterial communities, providing a mechanistic understanding of the stability of cooperative behaviours in complex multispecies communities.
Methods
Organisms and set-up of bacterial communities
We used the eight well-characterized Pseudomonas spp. genotypes CHA0, PF5, Q2-87, 1M1-96, Q8R1-96, MVP1-4, F113 and Phl1C2 (ref. 40). Although classified as P. fluorescens, recent comparative genomics studies indicate that they belong to at least three species41. Forty-eight communities were assembled from the eight genotypes, covering a richness of one (each of the eight genotypes in monoculture with two replicates each), two (16 combinations), four (12 combinations) and eight genotypes (4 replicates), according to a previously published design19. Also, these communities span a gradient in phylogenetic relatedness (see below). All genotypes were assessed for production of exoprotease as model public good. Bacteria were grown overnight in LB, pelleted by centrifugation and washed three times in 0.85% NaCl to remove nutrients. Bacteria were mixed to set-up the desired communities (total of 2 × 107 bacteria per community).
Emergence of evolved defectors
Bacteria were grown in QSM3, a minimal medium containing 1% bovine serum albumin as the sole carbon source, at 25 °C with agitation for 48 h. This time lapse corresponds to about 25 generations and is known to allow the evolution and spread of new phenotypes28. Bacteria were serial-diluted and enumerated on milk agar plates (tryptic soy broth 3 g l−1, agar 15 g l−1 and skim milk 150 ml l−1). In Pseudomonads, protease-negative phenotypes emerge as a consequence of deactivation of the gac–rsm QS cascade9,28. To confirm that ’defectors’ were not simply protease-deficient mutants, we looked for colonies lacking, in addition to proteolytic activity, tryptophane dioxygenase activity, another trait regulated by the gac–rsm pathway27. Three days old colonies were screened for proteolytic (transparent halo around the colony) and tryptophan dioxygenase activity (brown colouration when overlaid with tryptophane42). We counted colonies lacking exoproteases and tryptophan dioxygenase as signal-blind defectors27. In order to further test if these mutants are signal blind, we randomly picked 20 protease-deficient isolates and attempted to grow them in QSM medium with or without an organic extract from wild-type cultures (containing QS signals) as described elsewhere17.
Frequency-dependent fitness of signal-blind defectors
We used the isogenic signal-blind mutant CHA0ΔgacS, chromosomally tagged with green fluorescent protein43. This mutant lacks QS and lacks extracellular proteases required for growth on proteins30. Signal blindness is predicted to be the most advantageous defection strategy10, and is the most common mechanism of defection from QS-regulated traits in pseudomonads9. Forty-eight microbial communities were assembled as described above and mixed with the defector CHA0ΔgacS-green fluorescent protein at frequencies of 10%, 33%, 66% and 90% defector. The OD600 of the whole community including defector was adjusted to 0.05 in 200 μl QSM. A total of 192 communities were established (48 communities × 4 defector frequencies) and grown with agitation at 25 °C for 36 h (early stationary growth phase). At the beginning and at the end of the experiment, bacteria were enumerated using a C6 Flow Cytometer (Accuri). Total bacteria were gated on the base of their forward scatter (FSC) side scatter (SSC) signal, defectors on the base of the FL1-A signal (green fluorescence). The relative fitness ω of the defector was calculated from the ratio of Malthusian growth parameters: ω=ln (Xfinal/Xinitial)/ln(Yfinal/Yinitial), where X and Y are the concentrations (number of bacteria ml−1) of the defector and the remaining community, respectively3. Relative fitness >1 indicates superior growth of the defector.
Phylogenetic relationships
Phylogenetic distance was estimated on the base of the phlD nucleotide sequence, a functional gene coding for a polyketide synthase present in all tested genotypes. This gene provides a robust estimator of phylogenetic distance among the studied bacteria44 and correlates well with metabolic differentiation24. Genetic distance between the genotypes was calculated with the Kimura algorithm using the dna.dist() function (package ’ape’) in R. Mean genetic distance between the defector and the remaining community was defined as MGDx= , where ‘Dist’ is the genotypic distance between the defector x and the cooperator y, and P the relative abundance of y in the community. The mean pairwise genetic distance between genotypes present in a community was defined as MGDtot=
, where ‘Dist’ is the genotypic distance between the defector x and the cooperator y, and P the relative abundance of y in the community. The mean pairwise genetic distance between genotypes present in a community was defined as MGDtot= . MGDtot is equivalent to ‘genotypic dissimilarity’ in previous studies24. See Fig. 5 for a detailed view of the interrelationships between diversity indices and a neighbor-joining phylogenetic tree of the used genotypes.
. MGDtot is equivalent to ‘genotypic dissimilarity’ in previous studies24. See Fig. 5 for a detailed view of the interrelationships between diversity indices and a neighbor-joining phylogenetic tree of the used genotypes.
(a) phlD-based neighbour-joining tree of the eight used genotypes. (b) Relationship between community richness (number of genotypes) and the mean pairwise distance between the present genotypes and the model defector Pseudomonas fluorescens CHA0ΔgacS. (c) Relationship between the community richness and mean pairwise phylogenetic distance between the genotypes.
Public good and toxin production
We tested the ability of the studied genotypes grown alone or in mixture to inhibit the growth of the signal-blind mutant CHA0ΔgacS30 by establishing a total of 96 communities ranging from one to eight genotypes24. Each community was grown in QSM as described above, the bacteria were pelleted by centrifugation (15,000 g, 5 min) and the supernatant was sterile filtered. Growth inhibition was assessed by mixing a liquid culture of CHA0ΔgacS in 135 μl 1/10 LB (OD600=0.05) supplemented with 15 μl of sterile filtered supernatant. Controls received sterile albumin-free QSM. Toxicity was rated as the relative growth inhibition of the defector at 25 °C after 24 h. To ensure that the observed growth inhibition patterns were caused by signal blindness and not by the sensitivity of the parent genotype, we repeated the measurements with P. fluorescens CHA0 as test organism. Public goods (extracellular proteases) were defined as the proteolytic activity in sterile filtered supernatant of each community, as measured with an azocasein assay45.
Modelling of defector fitness
To test if the observed patterns of public goods and defector-inhibiting toxins may explain defector fitness, we added a defector inhibition function to an existing snowdrift model31. We modelled the relative payoff P of an individual x in a mixed community as P(x,y)=B(x+y)–Cb(x)–Ct(x)–E × T(y,x), with B the benefit function of the total investment in public goods, Cb and Ct the cost function for public goods and toxins, and T the toxicity function for toxins produced by y, showing an efficiency E against x. Considering that the toxins only affect the defector and that defectors neither produce public goods nor toxins (Cb(x)=Ct(x)=0), the payoff of the defector x can be simplified to P(x)=B(x+y)–E × T(y,x) and the payoff of the cooperator y to P(y)=B(x+y)–Cb(y)–Ct(y) (ref. 31). In order to reflect that QS-regulated traits are density-dependent13,14, we assume the per capita investment of the cooperator y to linearly increase with frequency, so that the B(y), Cb(y) and Ct(y) functions are quadratic functions of cooperator frequency. If E × T(y,x)>Cb(x)+Ct(x), the advantages of defecting are counterbalanced by inhibition, resulting in higher relative payoff of cooperators (P(x)−P(y)<0). At low defector frequency, cooperators will invest more in anti-defector toxins, which may harm rare defectors and cancel the benefits of defection. We explored different parameter combinations consistently showing a transition from a negative frequency-dependent to a hump-shaped defector fitness curve as E increases, indicating that rare defector mutants may be unable to invade communities of cooperating bacteria efficiently harming them (Fig. 3).
Statistical analyses
We analysed the effects of initial defector frequency (continuous), genotypic richness of the community of cooperators (categorical) and genetic distance between defector and the remaining community of cooperators (continuous) on defector fitness with a polynomial GLM. For more precision, we used the measured initial defector frequency in each single community and not the set-up one, justifying its use as continuous factor (see Fig. 2b). Using the same approach, the effect of the production of proteases (public good) and specific defector growth reduction (inhibition efficiency) on defector fitness was assessed. To account for the imperfect orthogonality between the diversity indices (Fig. 5), we fitted richness and mean phylogenetic distance in a sequential GLM. Sequential GLM is a common statistical approach to test several inter-related biodiversity measures in one model46. The effects of genotypic richness and genetic distance between the defector and the community of cooperators were further tested against bacterial composition in order to avoid pseudo-replication24,46.
Additional information
How to cite this article: Jousset, A. et al. Evolutionary history predicts the stability of cooperation in microbial communities. Nat. Commun. 4:2573 doi: 10.1038/ncomms3573 (2013).
References
West, S. A., Diggle, S. P., Buckling, A., Gardner, A. & Griffins, A. S. The social lives of microbes. Annu. Rev. Ecol. Evol. Syst. 38, 53–77 (2007).
Griffin, A. S., West, S. A. & Buckling, A. Cooperation and competition in pathogenic bacteria. Nature 430, 1024–1027 (2004).
Diggle, S. P., Griffin, A. S., Campbell, G. S. & West, S. A. Cooperation and conflict in quorum-sensing bacterial populations. Nature 450, 411–417 (2007).
Jousset, A. et al. Predators promote toxicity of rhizosphere bacterial communities by selective feeding on non-toxic cheaters. ISME J. 3, 666–674 (2009).
West, S. A., Griffin, A. S., Gardner, A. & Diggle, S. P. Social evolution theory for microorganisms. Nat. Rev. Microbiol. 4, 597–607 (2006).
Travisano, M. & Velicer, G. J. Strategies of microbial cheater control. Trends Microbiol. 12, 72–78 (2004).
Keller, L. & Surette, M. G. Communication in bacteria: an ecological and evolutionary perspective. Nat. Rev. Microbiol. 4, 249–258 (2006).
Darch, S. E., West, S. A., Winzer, K. & Diggle, S. P. Density-dependent fitness benefits in quorum-sensing bacterial populations. Proc. Natl Acad. Sci. USA. 109, 8259–8263 (2012).
van den Broek, D., Chin-a-Woeng, T. F. C., Bloemberg, G. V. & Lugtenberg, B. J. J. Molecular nature of spontaneous modifications in GacS which cause colony phase variation in Pseudomonas sp. Strain PCL1171. J. Bacteriol. 187, 593–600 (2005).
Czaran, T. & Hoekstra, R. F. Microbial communication, cooperation and cheating: quorum sensing drives the evolution of cooperation in bacteria. PLoS One 4, e6655 (2009).
Jiricny, N. et al. Fitness correlates with the extent of cheating in a bacterium. J. Evol. Biol. 23, 738–747 (2010).
Ross-Gillespie, A., Gardner, A., West, S. A. & Griffin, A. S. Frequency dependence and cooperation: theory and a test with bacteria. Am. Nat. 170, 331–342 (2007).
El Mouden, C., West, S. A. & Gardner, A. The enforcement of cooperation by policing. Evolution 64, 2139–2152 (2010).
West, S. A., Griffin, A. S. & Gardner, A. Evolutionary explanations for cooperation. Curr. Biol. 17, R661–R672 (2007).
Hamilton, W. D. Genetical evolution of social behaviour I. J. Theor. Biol. 7, 1–16 (1964).
Ostrowski, E. A., Katoh, M., Shaulsky, G., Queller, D. C. & Strassmann, J. E. Kin discrimination increases with genetic distance in a social amoeba. PLoS Biol. 6, 2376–2382 (2008).
Dubuis, C. & Haas, D. Cross-species GacA-controlled induction of antibiosis in pseudomonads. Appl. Environ. Microbiol. 73, 650–654 (2007).
Cordero, O. X. et al. Ecological populations of bacteria act as socially cohesive units of antibiotic production and resistance. Science 337, 1228–1231 (2012).
Becker, J., Eisenhauer, N., Scheu, S. & Jousset, A. Increasing antagonistic interactions cause bacterial communities to collapse at high diversity. Ecol. Lett. 15, 468–474 (2012).
Mitri, S., Xavier, J. B. & Foster, K. R. Social evolution in multispecies biofilms. Proc. Natl. Acad. Sci. USA 108, (Suppl 2): 10839–10846 (2011).
Vetsigian, K., Jajoo, R. & Kishony, R. Structure and evolution of Streptomyces interaction networks in soil and in silico. PLoS Biol. 9, e1001184 (2011).
Validov, S. et al. Antagonistic activity among 2,4-diacetylphloroglucinol-producing fluorescent Pseudomonas spp. FEMS Microbiol. Lett. 242, 249–256 (2005).
Rainey, P. B. & Rainey, K. Evolution of cooperation and conflict in experimental bacterial populations. Nature 425, 72–74 (2003).
Jousset, A., Schmid, B., Scheu, S. & Eisenhauer, N. Genotypic richness and dissimilarity opposingly affect ecosystem performance. Ecol. Lett. 14, 537–624 (2011).
Lapouge, K., Schubert, M., Allain, F. H. T. & Haas, D. Gac/Rsm signal transduction pathway of gamma-proteobacteria: from RNA recognition to regulation of social behaviour. Mol. Microbiol. 67, 241–253 (2008).
Duffy, B. K. & Defago, G. Controlling instability in gacS-gacA regulatory genes during inoculant production of Pseudomonas fluorescens biocontrol strains. Appl. Environ. Microbiol. 66, 3142–3150 (2000).
Bull, C. T. et al. Characterization of spontaneous gacS and gacA regulatory mutants of Pseudomonas fluorescens biocontrol strain CHA0. Antonie Van Leeuwenhoek 79, 327–336 (2001).
Martinez-Granero, F., Capdevila, S., Sanchez-Contreras, M., Martin, M. & Rivilla, R. Two site-specific recombinases are implicated in phenotypic variation and competitive rhizosphere colonization in Pseudomonas fluorescens. Microbiol. 151, 975–983 (2005).
Hibbing, M. E., Fuqua, C., Parsek, M. R. & Peterson, S. B. Bacterial competition: surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 8, 15–25 (2010).
Zuber, S. et al. GacS sensor domains pertinent to the regulation of exoproduct formation and to the biocontrol potential of Pseudomonas fluorescens CHA0. Mol. Plant-Microbe Interact. 16, 634–644 (2003).
Doebeli, M., Hauert, C. & Killingback, T. The evolutionary origin of cooperators and defectors. Science 306, 859–862 (2004).
Brown, D. A mathematical model of the Gac/Rsm quorum sensing network in Pseudomonas fluorescens. Biosystems 101, 200–212 (2010).
Manhes, P. & Velicer, G. J. Experimental evolution of selfish policing in social bacteria. Proc. Natl. Acad. Sci. USA 108, 8357–8362 (2011).
Frank, S. A. Mutual policing and repression of competition in the evolution of cooperative groups. Nature 377, 520–522 (1995).
Heeb, S., Valverde, C., Gigot-Bonnefoy, C. & Haas, D. Role of the stress sigma factor RpoS in GacA/RsmA-controlled secondary metabolism and resistance to oxidative stress in Pseudomonas fluorescens CHA0. FEMS Microbiol. Lett. 243, 251–258 (2005).
Ratnieks, F. L. W. & Helanterä, H. The evolution of extreme altruism and inequality in insect societies. Philos. Trans. R. Soc. B: Biol. Sci. 364, 3169–3179 (2009).
Jones, A. G., Arnold, S. J. & Burger, R. The mutation matrix and the evolution of evolvability. Evolution 61, 727–745 (2007).
West, S. A., Pen, I. & Griffin, A. S. Conflict and cooperation – cooperation and competition between relatives. Science 296, 72–75 (2002).
D' Antonio, C. M. & Vitousek, P. M. Biological invasions by exotic grasses, the grass fire cycle, and global change. Annu. Rev. Ecol. Syst. 23, 63–87 (1992).
de la Fuente, L., Mavrodi, D. V., Landa, B. B., Thomashow, L. S. & Weller, D. M. phlD-based genetic diversity and detection of genotypes of 2,4-diacetylphloroglucinol-producing Pseudomonas fluorescens. FEMS Microbiol. Ecol. 56, 64–78 (2006).
Loper, J. E. et al. Comparative genomics of plant-associated Pseudomonas spp.: insights into diversity and inheritance of traits involved in multitrophic interactions. PLoS Genet. 8, e1002784 (2012).
Oberhansli, T., Defago, G. & Haas, D. Indole-3-acetic-acid (IAA) synthesis in the biocontrol strain CHA0 of Pseudomonas fluorescens – role of tryptophan side-chain oxidase. J. Gen. Microbiol. 137, 2273–2279 (1991).
Jousset, A., Lara, E., Wall, L. G. & Valverde, C. Secondary metabolites help biocontrol strain Pseudomonas fluorescens CHA0 to escape protozoan grazing. Appl. Environ. Microbiol. 72, 7083–7090 (2006).
Moynihan, J. A. et al. Evolutionary history of the phl gene cluster in the plant-associated bacterium Pseudomonas fluorescens. Appl. Environ. Microbiol. 75, 2122–2131 (2009).
Sambrook, J. & Russell, D. Molecular Cloning: A Laboratory Manual Cold Spring Harbor Laboratory Press (2001).
Schmid, B. et al. inBiodiversity and Ecosystem Functioning: Synthesis and Perspectives eds Loreau M., Naeem S., Inchausti P. 61–75Oxford University Press (2002).
Acknowledgements
We are grateful to Angus Buckling and Will Ratcliff for helpful comments on this study.
Author information
Authors and Affiliations
Contributions
A.J. designed the experiments. E.M. and A.J. performed the experiments. N.E. and A.J. performed statistical analyses. A.J., N.E. and S.S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Jousset, A., Eisenhauer, N., Materne, E. et al. Evolutionary history predicts the stability of cooperation in microbial communities. Nat Commun 4, 2573 (2013). https://doi.org/10.1038/ncomms3573
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms3573
This article is cited by
-
A single mutation in rapP induces cheating to prevent cheating in Bacillus subtilis by minimizing public good production
Communications Biology (2018)
-
Soil microarthropods alter the outcome of plant-soil feedback experiments
Scientific Reports (2018)
-
Where less may be more: how the rare biosphere pulls ecosystems strings
The ISME Journal (2017)
-
Phylogenetic relatedness determined between antibiotic resistance and 16S rRNA genes in actinobacteria
BMC Microbiology (2015)
-
Quorum sensing enhancement of the stress response promotes resistance to quorum quenching and prevents social cheating
The ISME Journal (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.