Abstract
The ubiquitin ligase SCFCdc4 mediates phosphorylation-dependent elimination of numerous substrates by binding one or more Cdc4 phosphodegrons (CPDs). Methyl-based NMR analysis of the Cdc4 WD40 domain demonstrates that Cyclin E, Sic1 and Ash1 degrons have variable effects on the primary Cdc4WD40 binding pocket. Unexpectedly, a Sic1-derived multi-CPD substrate (pSic1) perturbs methyls around a previously documented allosteric binding site for the chemical inhibitor SCF-I2. NMR cross-saturation experiments confirm direct contact between pSic1 and the allosteric pocket. Phosphopeptide affinity measurements reveal negative allosteric communication between the primary CPD and allosteric pockets. Mathematical modelling indicates that the allosteric pocket may enhance ultrasensitivity by tethering pSic1 to Cdc4. These results suggest negative allosteric interaction between two distinct binding pockets on the Cdc4WD40 domain may facilitate dynamic exchange of multiple CPD sites to confer ultrasensitive dependence on substrate phosphorylation.
Similar content being viewed by others
Introduction
WD40 domains, and the related Kelch β-propeller domains, mediate protein interactions in many contexts and together form the largest domain family in the human genome1. WD40 domain proteins are a predominant class of substrate receptor in the ubiquitin-proteasome system, found in F-box proteins and other subunits of the cullin-RING (CRL) ubiquitin ligases2,3,4. The WD40 domain of the yeast F-box protein Cdc4 (Cdc4WD40) recognizes substrates via phosphorylated motifs termed Cdc4 phosphodegrons (CPDs). One of the best characterized Cdc4 substrates is the cyclin dependent kinase inhibitor Sic1, which is targeted for ubiquitination and degradation in late G1 phase upon its phosphorylation by Cln-Cdc28 kinase activity5,6. Sic1 is phosphorylated at 9 low-affinity CPD sites (Kd ∼10-100 μM) that all bear mismatches to the optimal CPD sequence (I/L-I/L/P-pT-P-
Nuclear magnetic resonance (NMR) analysis suggests that multiple Sic1 low-affinity CPD sites interact with Cdc4 in a dynamic equilibrium to generate an overall high-affinity interaction13,14. The structure of a high-affinity singly phosphorylated CPD bound to Cdc4 revealed that the P0 phosphorylated residue occupies a deep arginine-rich pSer/Thr binding pocket in the WD40 domain, and that adjacent sub-pockets confer sequence selectivity at P−2, P−1, P+1 and P+2 CPD positions7. A distal basic surface in Cdc4 and its human orthologue Fbw7 can also simultaneously engage a second phosphorylated residue at the P+4 position to increase affinity15,16,17, or conversely antagonize the interaction of CPDs with C-terminal basic residues7. However, this secondary phosphate-binding site is dispensable for Sic1 degradation in vivo8. Dimerization of the SCFFbw7 complex also contributes to the recognition of some multisite phosphorylated substrates18, and catalytic efficiency of the SCFCdc4 complex19. Cln- and Clb-CDK may act in a processive multi-phosphorylation cascade on Sic1 to facilitate Cdc4 recognition through generation of appropriate CPD sites16. Recognition of the Ash1 transcriptional repressor by Cdc4 depends on three closely-spaced CPD sites that form a redundant diphosphodegron20. Other substrates, including Far1, Gcn4, Cln2 and Cln3 for Cdc4 in yeast and cyclin E, c-Myc and Mcl1 for Fbw7 in humans, also may depend on multisite phosphorylation2,21,22. These various interaction modes illustrate the tunability of the Cdc4/Fbw7 system for substrate elimination.
The phenomenon of allostery, whereby ligand binding at one site alters interactions at a physically distant site, allows for complex control of protein function23,24,25. A biplanar dicarboxylic acid compound called SCF-I2 allosterically inhibits Cdc4WD40-substrate interactions by binding to a site 25 Å away from the main CPD pocket26. SCF-I2 elicits this allosteric effect through structural changes that occlude the P−2 region of the CPD pocket. Enigmatically, SCF-I2 is 10-fold less potent towards full-length Sic1 compared to a singly phosphorylated high-affinity CPD of similar affinity for Cdc4. The SCF-I2 site and associated allosteric infrastructure appear to be conserved through evolution27.
The large size of the Cdc4 complex previously precluded determination of substrate interaction effects on the WD40 domain. Here we investigate substrate-Cdc4 interactions by NMR analysis of assigned methyl groups in the isolated Cdc4WD40 domain. Unexpectedly, we find that the CPDs of an extended phosphorylated Sic1 substrate directly contact the SCF-I2 allosteric site. On the basis of this data and the inhibitory effect of SCF-I2 on the CPD pocket, we predicted that extended multisite CPDs might engage the WD40 domain through both pockets in a mutually antagonistic manner, and then confirmed this hypothesis using fluorescence-binding data. Consistent with this negative allosteric effect, NMR analysis reveals smaller chemical shifts in the P−2 sub-pocket for extended multisite CPDs compared with short CPD-containing peptides. Mathematical modelling suggests that interactions at the primary and allosteric pockets may enhance the ultrasensitive dependence of the Sic1-Cdc4 interaction on Sic1 phosphorylation. This negative allosteric binding mechanism helps explain the conundrum of how multiple low-affinity sites can generate a rapidly exchangeable yet high-affinity Sic1-Cdc4 interaction.
Results
Characterization of isolated Cdc4WD40
We determined the effects of substrate binding on the isolated WD40 domain of Cdc4 (Cdc4WD40), which is more amenable to NMR due to its size (40 kDa) than the minimal Cdc4-Skp1 complex (∼70 kDa). The N-terminal targeting region of Sic1 (residues 1-90, called Sic11-90), which contains 7 of the 9 Sic1 CPD sites and is sufficient for Cdc4 binding28, was phosphorylated to completion by Cln2-Cdc28-Cks1 kinase (called pSic1). We used isothermal titration calorimetry (ITC) to determine the affinity of Cdc4WD40 for pSic1 and SCF-I2 (Supplementary Fig. 1a–c). The Kd value for pSic1-Cdc4WD40 was 0.8±0.12 μM, similar to the 0.6 μM value determined for pSic1-Cdc4-Skp1 by intrinsic tryptophan (Trp) fluorescence13. The Kd value for SCF-I2 binding Cdc4WD40 was 0.6±0.02 μM, concordant with the ∼2 μM Ki value determined for SCF-I2-Cdc4-Skp1 by a fluorescence polarization competition assay26.
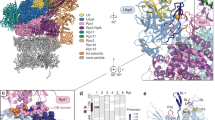
NMR resonances for Cdc4WD40 were highly broadened, with millisecond–microsecond timescale motion likely playing a significant role. Although addition of a CycE phosphopeptide or SCF-I2 sharpened resonances (Supplementary Fig. 1d,e), backbone assignment was not possible. However, as Cdc4WD40 methyl resonances were sharper due to the favourable properties of methyls29,30, we analysed Ile-δ1-[13CH3] and Met-ɛ-[13CH3] (IM)-labelled samples (Fig. 1a). Cdc4WD40 contains 24 Ile and 5 Met residues distributed throughout the WD40 domain. Three Ile (Ile573, Ile716 and Ile385) and 1 Met (Met590) are adjacent to the arginine-rich CPD binding pocket on Cdc4 and another 9 Ile residues lie in close proximity to both the CPD and the allosteric pockets. Point mutations of 15 Ile to Val or Met to Val enabled assignment of 12 Ile and 1 Met methyls in Cdc4WD40 (Fig. 1b).
(a) 1H–13C TROSY HMQC spectrum of Cdc4WD40 with assigned Ile and Met resonances. (b) Surface and ribbon representation of Cdc4WD40 with CycE peptide (PDB ID 1NEX) in yellow, CPD-binding pocket7 in blue, SCF-I2 binding pocket26 in cyan and assigned residues in red spheres. (c) and (e) Combined 1H and 13C chemical shift perturbations of Cdc4WD40 upon (c) CycE or (e) pSic1 binding. Assigned Ile and Met residues are in red, labelled with residue number; unassigned residues in pink. (d) and (f) Surface and ribbon representation of Cdc4WD40 with (d) CycE peptide (1NEX) or (f) Sic1 peptide (3V7D). Colouring as for (b) except only residues showing significant chemical shift changes are highlighted in red spheres.
Perturbation of Cdc4WD40 methyl resonances
Unlabelled CycE phosphopeptide, pSic1 or various phosphopeptides derived from Sic1 and Ash1 were titrated into IM-labelled Cdc4WD40 samples and chemical shift perturbations and changes in line shape were observed as a function of ligand concentration. Ligand interactions are expected to broaden NMR spectra due to slowed tumbling, exchange between free and protein/peptide-bound states, and dynamic exchange within the bound state (Supplementary Note 1). Upon titration of CycE phosphopeptide into IM-labelled Cdc4WD40, large chemical shift perturbations (>20 Hz combined 1H/13C shifts, see Methods, Fig. 1c,d, Supplementary Fig. 2a) were observed for Ile396P, Ile573P, Met590P and Ile716P (where P indicates a residue close to the primary CPD binding pocket) and Ile654 (which lies between the primary CPD and allosteric inhibitor pockets). We also observed a smaller but still significant chemical shift perturbation on Ile385P. These NMR results are consistent with engagement of the primary CPD pocket by CycE (Fig. 1d).
Titration of unlabelled pSic1 into IM-labelled Cdc4WD40 resulted in large chemical shift perturbations for residues Ile385P, Ile396P and Met590P in the primary CPD pocket, as for CycE. Perturbations around the CPD pocket induced by pSic1 matched expectations from X-ray structures of CycE and pSic1 phosphopeptide-Cdc4 complexes7,8 (Fig. 1d,f). We also observed differences for pSic1 binding compared to CycE, including smaller chemical shift perturbation for Ile716P and no change for Ile573P. In contrast to CycE, pSic1 elicited extensive chemical shift perturbations on blade 5 (Ile577A, Ile594A and Ile596A, where A indicates a residue close to the allosteric inhibitor binding pocket) and blade 6 (Ile654) of Cdc4WD40, precisely around the SCF-I2 allosteric binding site (Fig. 1f; Supplementary Fig. 2b).
To deconvolute effects of the multiple CPDs in pSic1, short phosphopeptides (Sic110pT2/pT5, Sic19pT45, Sic19pT173 and Sic120pS69/pS76/pS80) were titrated into IM-labelled Cdc4WD40. Each perturbed methyl chemical shifts in the primary pocket (Supplementary Fig. 3), as for CycE. However, only the longer Sic120pS69/pS76/pS80 phosphopeptide perturbed chemical shifts on blade 5 (Ile594A, Ile596A, Ile577A). These data suggested either that in the context of an extended multisite substrate suboptimal CPD sequences affect the SCF-I2 binding pocket through allosteric transmission from the primary CPD pocket or that the CPDs directly contact the SCF-I2 binding pocket.
The allosteric binding pocket directly engages pSic1
We determined that all allosteric pocket residues exhibited fast-to-intermediate timescale exchange dynamics (Fig. 2a,b), consistent with weak direct interaction, unlike the slow-intermediate exchange observed for some residues in the primary CPD pocket (see below). To identify potential contact residues between pSic1 and Cdc4WD40, we performed methyl cross-saturation experiments. Among the 13 assigned Ile and Met methyl resonances, only Ile396P, Ile594A, Ile596A and Ile654 intensities were decreased significantly upon addition of pSic1 (Fig. 2c). Because cross-saturation exploits fast exchange between free and bound states31, we did not observe significant intensity changes for residues close to the primary CPD pocket due to slow exchange (Supplementary note 1). However, residues around a positively charged surface that includes Arg655 and Arg664 of the allosteric pocket were susceptible to cross-saturation (Fig. 2d,e). These results unambiguously identify the allosteric site as a direct second binding pocket on Cdc4WD40 for CPD motifs, and eliminate the alternative possibility of indirect allosteric effects transmitted from binding at the primary CPD pocket.
(a) Surface representation of Cdc4WD40 with Sic1 peptide in yellow and residues in the allosteric pocket in red spheres. (b) 1H–13C TROSY HMQC spectra of residues (i) Ile594 and (ii) Ile596 in the absence (red) and presence of increasing amounts 50% (orange), 75% (green), 120% (blue), 200% (purple) of pSic1, (iii) intensity ratios of the peaks corresponding to the unbound states and (iv) chemical shifts of the peaks corresponding to the bound states at different saturation levels. (c) Ratio of signal intensities originating from Ile and Met methyl groups with and without irradiation in the cross-saturation experiments. Intensity ratio of 1 is depicted as black line, one standard deviation from mean is depicted as dashed line. (d-e) Surface representation of Cdc4WD40 indicating electrostatic potential. Blue and red indicate regions of positive and negative potential, respectively; residues showing significant cross-saturation effect are in green spheres; Sic1 peptide is in yellow. (f) Combined 1H and 13C chemical shifts of Cdc4WD40 on SCF-I2 binding. The assigned Ile and Met residues are in red, labelled with residue number; unassigned residues in pink. (g) Surface and ribbon representation of Cdc4WD40 with SCF-I2 (3MKS). SCF-I2 is in yellow, SCF-I2 binding pocket in cyan and residues showed significant chemical shift changes are in red spheres.
Arg655, Arg664 and Trp657 are the main determinants of SCF-I2 binding in the allosteric pocket26. Side chains of these residues form salt bridges and hydrogen bonds with the two SCF-I2 carboxylic acids and may likewise engage phosphates. The allosteric pocket appears to be contacted only by extended multisite phosphopeptides (pSic1, Sic120pS69/pS76/pS80), but not short Sic110pT2/pT5, Sic19pT45, Sic19pT173 phosphopeptides (Supplementary Fig. 3). We speculated that tethering of an extended substrate at the primary CPD pocket may increase local CPD concentration and drive interactions at the weaker-affinity allosteric site32. We used the program HADDOCK33 to dock Sic120pS69/pS76/pS80 onto the allosteric pocket based on chemical shift perturbations and cross-saturation. One of the main simulated orientations placed one CPD in the allosteric pocket with potential contacts of another CPD at the primary binding pocket (Supplementary Fig. 4a,b).
IM-labelled Cdc4WD40 was also titrated with unlabelled SCF-I2. We observed large chemical shift perturbations close to the SCF-I2 binding pocket (Ile594A, Ile596A, Ile654 and Ile676) and also adjacent to the P−2 sub-pocket of the CPD binding interface (Met590P and Ile573P; Fig. 2f,g). These data confirm long-range structural effects on the CPD binding pocket caused by insertion of the inhibitor at the allosteric pocket26. SCF-I2 also had a smaller but still significant effect on Ile385P and Ile716P next to the P−1 sub-pocket, not evident in the SCF-I2 bound crystal structure26. These results demonstrate that pSic1 and SCF-I2 have an overlapping binding surface on Cdc4WD40 and would therefore be expected to compete for binding at this position34.
Role of the allosteric pocket in multisite recognition
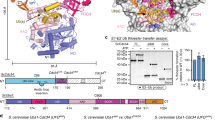
We assessed the contribution of the allosteric pocket to substrate interactions by mutation of positively charged Arg655 and Arg664 residues, both of which are required for SCF-I2 inhibition26. We generated Arg655Gln and Arg664Gln substitution mutants to minimize disruptive steric effects on the allosteric pocket and WD40 domain. Mutation of either Arg residue resulted in four- to fivefold reduction in pSic1-Cdc4WD40 affinity by in vitro Trp fluorescence (Fig. 3a, Table 1). Importantly, these mutations did not significantly reduce CycE-Cdc4WD40 affinity, consistent with CycE not appreciably interacting with the allosteric pocket (Fig. 1c).
(a) Trp fluorescence-binding curves for interactions of pSic1 and wild-type (black), Arg664Gln (cyan) and Arg655Gln Cdc4WD40 (magenta). (b) Morphology of yeast strains bearing wild-type or allosteric site mutant alleles of CDC4 transformed with a pGAL1-SIC1 plasmid and grown overnight on 2% galactose medium. Results are shown for the both W303 (Arg655Gln, Arg664Gln, Arg655GlnArg664Gln) and S288C (Arg655Ala and Arg664Ala) strain backgrounds. Error bars indicate s.d. for triplicate independent measurements. Scale bars, 10 μm (c) DNA content of wild-type and allosteric site mutant alleles of Cdc4 for the same strains shown in b.
Previous mutational analysis of the allosteric pocket showed that Arg655Ala and Arg664Ala substitutions completely block SCF-I2 inhibition in vitro, but do not cause obvious growth or cell cycle defects in vivo26. As other nominally functional Cdc4 mutants are sensitive to Sic1 dosage and phosphorylation status8, we examined effects of additional Sic1 dosage on Arg655 and Arg664 mutants. While SIC1 overexpression had little impact on mutant strain growth rates (Supplementary Fig. 4g), additional SIC1 dosage caused accumulation of cells with elongated buds and a concomitant G1 delay (Fig. 3b,c), consistent with a mild Sic1 elimination defect. These results suggest that, while not essential, the allosteric pocket facilitates Cdc4 substrate degradation in vivo.
Allosteric pocket binding weakens the primary CPD interaction
We compared affinities of Sic1 peptides phosphorylated either on Ser69 (Sic120pS69) or Ser80 (Sic120pS80) alone or together (Sic120pS69/pS80). NMR titration of the Sic120pS69/pS76/pS80 phosphopeptide perturbed chemical shifts in the primary and allosteric pockets (Supplementary Fig. 3d), suggesting that Ser69- and/or Ser80-containing CPDs can bind both pockets (Fig. 4a,b) and that the two CPDs are distant enough to enable simultaneous binding (Fig. 4c). To test this model, we computationally docked the Sic120pS69/pS80 phosphopeptide onto Cdc4WD40 at the primary and allosteric pockets, and found that the two sites can indeed simultaneously engage with no obvious energetic strain (Supplementary Fig. 4c).
(a–c) Possible binding states for (a) Sic120pS69, (b) Sic120pS80 and (c) Sic120pS69/pS80 peptides to wild-type Cdc4WD40 and mutant (Arg664Gln) Cdc4WD40. (d-f) Schematic of the extent of allosteric effects caused by binding of (d) pSic1, (e) CycE peptide and (f) Sic120pS69/pS76/pS80. For pSic1 (d), binding of one of the CPDs in pSic1 (P0) to the allosteric pocket (labelled A in cyan) between blade 5 and 6 induces rotation of Tyr574 (red arrows) and impairs binding in the P-2 sub-pocket (grey rectangle), demonstrated by the absence of chemical shift perturbation on Ile573 (grey spheres) on pSic1 binding. CycE peptide (e) does not bind the allosteric pocket, suggested by lack of chemical shift perturbation on residues around the allosteric pocket (grey spheres), and thus binding in the P-2 sub-pocket remains intact (red rectangle) and chemical shift perturbation was observed for Ile573 (red spheres). Binding of one of the CPDs in Sic120pS69/pS76/pS80 (f) to the allosteric pocket, suggested by the chemical shift perturbation on the residues around the allosteric pocket (red spheres), has a smaller effect on the binding in the P-2 sub-pocket (red dashed arrows) compared to pSic1, demonstrating some engagement. The chemical shift perturbation for Ile573 on binding Sic120pS69/pS76/pS80 peptide (light red spheres) is smaller than on binding CycE peptide (or other Sic1 peptides).
We determined phosphopeptide binding affinities for wild-type and mutant (Arg664Gln) Cdc4WD40 by Trp fluorescence (Supplementary Fig. 4d–f). Strikingly, the association constant for Sic120pS69/pS80 binding to wild-type Cdc4WD40, Ka(p69,p80|wt)=1.42 × 105 M−1, was about half that for Sic120pS69/pS80 binding to mutant Cdc4WD40, Ka(p69,p80|mt)=2.67 × 105 M−1 (see Methods, Table 2). Despite the presence of two binding pockets on wild-type Cdc4WD40, binding was considerably weaker than for Cdc4WD40 bearing only the primary CPD pocket. This result demonstrated negative allostery for a diphosphopeptide with sites that are sufficiently spaced to allow simultaneous contact with the primary and allosteric pockets. In contrast, if Cdc4WD40 had two independent binding sites, the expected result would be higher affinity for the wild-type due to additive contributions of both sites. We also found that the Sic120pS69/pS80 Ka(p69,p80|wt)=1.42 × 105 M−1 was smaller than the sum Ka(p69|wt)+Ka(p80|wt)=2.07 × 105 M−1 for singly phosphorylated peptides binding to wild-type Cdc4WD40. This result is also in agreement with negative allosteric coupling between the primary and allosteric pockets, although other effects that weaken combinatorial interactions at different sites may also be operative32. Note that the negative effect of the allosteric site on Sic120pS69/pS80 diphosphopeptide binding is opposite to the decreased affinity of the allosteric site mutants for the larger pSic11-90 ligand. This apparent inconsistency is resolved by accounting for long-range electrostatic contributions from the four additional CPDs in pSic11-90 (see Supplementary note 4). Finally, we found that the doubly phosphorylated peptide (Sic120pS69/pS80) bound mutant Cdc4WD40 with Ka(p69,80|mt)=2.67 × 105 M−1, approximately 1.7 times larger than the sum Ka(p69|mt) +Ka(p80|mt)=1.56 × 105 M−1 for the singly phosphorylated peptides. This result is consistent with additional long-range electrostatic contributions from a second CPD in the absence of direct contact with Cdc4 (ref. 35).
Chemical shift perturbations in the primary CPD pocket
We then examined NMR chemical shift and line-shape perturbations for signatures of negative allostery. Although population-weighted averaging and differential broadening due to exchange between states prohibits quantitative interpretation, qualitative comparisons are possible. First, we examined residues near the P−1 sub-pocket lined by Trp717 and Val384 (Supplementary Fig. 5a). CycE led to Ile385P chemical shift changes dependent on peptide concentration (fast exchange), whereas two peaks were observed for Ile716P (slow-intermediate exchange; Supplementary Fig. 5b). The opposite was seen for pSic1 with Ile385P in slow-intermediate and Ile716P in fast exchange (Supplementary Fig. 5c). These chemical shift perturbations demonstrate binding of both CycE and pSic1 in the P−1 sub-pocket, albeit with some differences.
A striking difference between pSic1 and CycE was evident for Met590P and Ile573P close to the P−2 sub-pocket that binds hydrophobic residues of the CPD (Fig. 5a). For both CycE and pSic1, two peaks were observed for each residue reflecting slow-intermediate exchange between free and bound states (Fig. 5b,c). However, the complexity of the NMR line-shapes suggested a dynamic equilibrium, possibly including partially engaged states. Met590P is one of the most exposed side chains near the CPD pocket (Fig. 5a) and may be a general probe of substrate interactions. The loss of intensity and chemical shift perturbations for Met590P in both CycE and pSic1 titrations were similar. In contrast, Ile573P lies at the bottom of the P−2 sub-pocket and should be sensitive to deep engagement (Fig. 5a). While Ile573P was broadened and showed chemical shift changes in the presence of CycE (Fig. 5b), neither effect was significant for pSic1 (Fig. 5c). These results suggest that the P−2 sub-pocket interacts less with pSic1 than CycE.
(a) Surface representation of Cdc4WD40 with Sic1 peptide in yellow and residues of the P-2 pocket in red spheres. (b,c) 1H–13C TROSY HMQC spectra of residues (i) Met590 and (ii) Ile573 in the absence (red) and presence of increasing amounts (50% (orange), 75% (green), 120% (blue), 200% (purple)) of (b) CycE peptide or (c) pSic1, (iii) intensity ratios of the peaks corresponding to the unbound states and (iv) chemical shifts of the peaks corresponding to the bound states at different saturation levels expressed as a percentage of bound substrates at the given Cdc4 to substrate molar ratio.
To investigate whether the apparent differential engagement of the P−2 pocket by pSic1 and CycE was due to the absence of preferred hydrophobic residues in P−2 positions of 8 of the 9 CPD sites of pSic1, titrations of pSic1 phosphopeptides (Sic110pT2/pT5, Sic19pT45, Sic19pT173 and Sic120pS69/pS76/pS80) were analysed. The binding of all phosphopeptides perturbed chemical shifts of Ile573P (Supplementary Fig. 3), as for CycE. The Sic120pS69/pS76/pS80 phosphopeptide actually had the smallest Ile573P perturbation, about one third that of CycE. This was surprising since the Leu at the P−2 position of the pSer76 site is a match to the Leu/Ile consensus; if the P−2 position were the sole determinant of binding, we might expect the Sic120pS69/pS76/pS80 phosphopeptide to have the largest Ile573P perturbation yet this was not the case. From these results, we concluded that the lack of consensus Leu/Ile residues in P−2 positions of pSic1 CPDs cannot explain the apparent differences in pSic1 and CycE binding the P−2 pocket, and instead suggested an allosteric effect might be at play.
The allosteric inhibitor SCF-I2 is known to cause occlusion of the P−2 sub-pocket due to re orientation of a main chain Tyr residue26. Both pSic1 and Sic120pS69/pS76/pS80 significantly perturbed residues on blade 5 (Ile594A, Ile596A and Ile577A) and also caused unexpectedly small chemical shift perturbations at Ile573P relative to CycE. We speculate that this NMR effect reflects decreased binding at the P−2 sub-pocket due to negative allosteric modulation of the primary CPD pocket upon simultaneous binding of another CPD site in the extended Sic1 multisite degrons (Fig. 4d–f).
Interactions of Ash1 with Cdc4WD40
We then investigated binding of IM-labelled Cdc4WD40 to unlabelled phosphopeptides derived from Ash1 residues 283-299. Singly phosphorylated peptides around pThr286, pThr290 and pSer294 sites have affinities for Cdc4 of 0.9, 4.1 and 6.5 μM, respectively, and the triphosphorylated peptide has an affinity of 0.07 μM (ref. 20). Ash19pT290 significantly perturbed residues close to the primary CPD pocket but not residues near the allosteric pocket (Fig. 6a,b). Ash19pS294 had much less effect on the P−1 sub-pocket than Ash19pT290 since Ile385P and Ile716P did not show significant perturbations (Fig. 6a,c). Since the P−1 and P−2 positions for the Thr290 and Ser294 sites match the CPD consensus (Supplementary Fig. 2c), the observed difference for the P−1 sub-pocket likely reflects the weaker affinity of pSer versus pThr for the P0 sub-pocket6. Consistently, a pSic1 phosphopeptide containing pSer at P0 (Sic120pS69/pS76/pS80) also minimally perturbed the P−1 sub-pocket (Supplementary Fig. 3).
(a) Combined 1H and 13C chemical shifts of Cdc4WD40 on binding of Ash1 peptides, with pT286 peptide in red, pT290 peptide in green, pS294 peptide in yellow, and the peptide phosphorylated on all three CPDs in blue. Assigned Ile and Met residues are labelled with residue number. Chemical shifts for the two bound states in titration with pT286 and triply phosphorylated peptides are reported on the same bars with stripes or without stripes. (b–e) Surface and ribbon representation of Cdc4WD40 coloured as in Fig. 1b except only residues showing significant chemical shift changes upon (b) pT290 peptide, (c) pS294 peptide, (d) pS286 peptide and (e) triply phosphorylated Ash1 peptide binding are displayed in red spheres. (f) Fluorescence polarization of the fluorescein-labelled Ash1 peptides (circle, pT286/pT290/pS294, square, pT286/pT290, triangle, pT290/pS294, inverted triangle doubly phosphorylated CycE peptide) in the presence of increasing concentrations of the recombinant Skp1-Cdc4 complex (mP, millipolarization unit). (g) Fluorescence polarization of the fluorescein-labelled Ash1 peptides (circle, pT286/pT290/pS294, square, pT286/pT290, triangle, pT290/pS294, inverted triangle, doubly phosphorylated CycE peptide) in the presence of increasing concentrations of the SCF-I2 inhibitor.
In contrast to the lower affinity Ash1 CPD motifs, Ash19pT286 led to two peaks for Ile385P, Ile396P, Ile573P, Ile586A, Ile594A and Ile596A resonances in the fully saturated complex, diagnostic of two bound-state conformations (Supplementary Fig. 6a). In one of these, chemical shift perturbations for Ile573P, Ile594A and Ile596A were much greater than for other residues (Fig. 6a,d). Titration with the Ash118pT286/pT290/pS294 triphosphopeptide resulted in similar chemical shift perturbations as for Ash19pThr286 but with slightly smaller shifts for Ile396P and Ile586A (Fig. 6a,e, Supplementary Fig. 6b). The magnitude of shifts for residues in the allosteric pocket for these Ash1 peptides was significantly larger than for pSic1 (Fig. 1e), suggesting that the short pThr286 CPD had greater direct effects on the primary CPD pocket and also indirect effects on the allosteric pocket.
Differential effects of SCF-I2 on Ash1 CPDs
Using fluorescence polarization26, we found that SCF-I2 was 10-fold more potent for inhibiting Ash118pT290/pS294 binding than Ash118pT286/pT290 or Ash118pT286/pT290/pS294 peptides (Fig. 6f,g, Table 3). However, since the Ash118pT286/pT290/pS294 bound equally well to wild-type versus Arg655Gln or Arg664Gln Cdc4WD40 (Supplementary Fig. 6c), we concluded the pThr286 CPD exerted an indirect allosteric effect on the SCF-I2 binding pocket. HADDOCK docking of Ash1 phosphopeptides to Cdc4WD40 based on chemical shift perturbations was used to provide a model for allosteric transmission. In these models, the P−2 sub-pocket of Cdc4 was occupied by the P−2 prolines of pThr290 and pSer294 CPDs but by the P−3 tryptophan of the pThr286 CPD, possibly due to predicted pi–pi interactions with Tyr594 (Supplementary Fig. 6d). Since SCF-I2 binding causes residue rearrangements near the CPD pocket, including Leu634, Val 635 and Tyr594 (Supplementary Fig. 6e), interaction between the P−3 tryptophan and Tyr594 may inhibit such rearrangements, thereby more effectively outcompeting SCF-I2 than other CPDs. These prominent indirect effects of the pThr286 Ash1 CPD on the allosteric pocket are in contrast to the minimal effects of CycE on the allosteric site and the direct allosteric site contacts made by CPDs in extended multisite ligands. The contributions of the allosteric pocket in Cdc4 may thus be modulated in a substrate-specific manner.
Biophysical model for dynamic site exchange
The above results suggested that dynamic exchange of the CPDs may be facilitated through negative allosteric communication between the primary and allosteric pockets. Binding of one CPD to the primary pocket would tether other CPDs in close proximity to Cdc4, and thereby enhance interactions with the low-affinity allosteric pocket (Fig. 7a, i and ii). In contrast to simple two-site binding, however, CPD binding to the allosteric pocket also weakens the CPD interaction with the primary pocket (Fig. 7a, iii), facilitating disassociation and subsequent rebinding to the primary pocket, possibly involving a previously non-engaged CPD (Fig. 7a, iv). Notably, engagement at the allosteric pocket provides a physical basis for tethering of Sic1 to Cdc4 upon transient disengagement from the primary CPD site.
(a) Interaction of one of the CPD motifs with the high-affinity, primary (P) CPD-binding pocket (dark blue) (i) facilitates the binding of another CPD site to the lower affinity allosteric pocket (A) (cyan) (ii). Binding to the allosteric pocket weakens the engagement of pSic1 in the P-2 sub-pocket via allosteric effects (iii) and increases the probability of the rebinding of another CPD site in the primary pocket (iv). (b) Schematic of a possible mechanism that enhances ultrasensitivity by involving both the primary (P) and allosteric (A) binding pockets on Cdc4 (solid ellipse) in its interaction with Sic1 (chain in dashed envelope, negatively charged phosphorylated CPDs and positively charged residues are represented, respectively, by red and blue circles). The Sic1-Cdc4 bound state comprises three interconverting configurations: only P engaged, only A engaged (top drawings), and both P and A engaged (bottom drawing). The Sic1 conformations of the transiently bound configuration with both P and A engaged simultaneously are envisioned to be spatially closer to Cdc4, on average (bottom drawing), than when only P or only A is engaged (top drawings). (c) Fractional bound ligand θ versus number of phosphorylation n predicted by the mathematical model. The average Sic1-Cdc4 distance is taken to be  =12 Å for the P-only or A-only configuration. The ultrasensitive transition is sharper when both P and A can be engaged (colour curves) than if only P can be engaged (black curve). The sharpness of the transition increases with decreasing average Sic1-Cdc4 distance
=12 Å for the P-only or A-only configuration. The ultrasensitive transition is sharper when both P and A can be engaged (colour curves) than if only P can be engaged (black curve). The sharpness of the transition increases with decreasing average Sic1-Cdc4 distance  = 8 Å (blue), 6 Å (green) and 5 Å (red) for the bound configuration with both P and A engaged simultaneously (bottom drawing in b).
= 8 Å (blue), 6 Å (green) and 5 Å (red) for the bound configuration with both P and A engaged simultaneously (bottom drawing in b).
In an earlier model, we rationalized the physical basis for ultrasensitivity by through-space long-range interactions between a single positively charged binding pocket on Cdc4 and the collection of negatively charged phosphates on Sic1 (ref. 35). Given evidence for CPD engagement of the allosteric pocket, we sought to ascertain whether the hypothesized polyelectrostatic basis for ultrasensitivity would hold. We extended our mathematical model to three bound configurations, under the same simplifying assumption of equivalent CPD motifs as the original model35 (Fig. 7b, Supplementary Fig. 7; see Supplementary note 3 for mathematical formulation). We refer to the single-point bound configurations with only the primary (P) pocket or only the allosteric (A) pocket engaged as P and A′, respectively, and the two-point bound configuration with both pockets engaged simultaneously as (P′,A). In this model, despite conformational entropy loss for two-point binding36, the (P′,A) configuration becomes more favourable than P or A′ when the Sic1 net charge becomes negative because the average Sic1 to Cdc4 distance for (P′,A) is smaller due to the constraint imposed on the Sic1 conformational ensemble by two-point binding, resulting in stronger electrostatic attraction between Cdc4 and non-contacting CPDs. This two-point contact model can lead to sharper ultrasensitive binding transitions than single-site models even though the transition midpoint remains essentially identical (Fig. 7c, Supplementary Fig. 7a). The critical role of non-contacting CPDs is underscored by considering an alternate model without long-range electrostatic contributions to binding, in which case ultrasensitivity is lost (Supplementary Fig. 7b). Note that the enhancement of ultrasensitivity by two-site binding does not rely on a precise degree of positive or negative allosteric coupling between the two Cdc4 sites but instead depends only on the existence of a two-site bound state to tether the Sic1 conformational ensemble closer to Cdc4 ( ). Negative coupling is, however, required for a similar transition midpoint in the one- and two-site models (Supplementary Fig. 7c). Comparing ultrasensitivity for different degrees of negative coupling between the two Cdc4 binding pockets, we found that as long as the bound Sic1 conformations are held closer on average to the Cdc4 for two-point than for one-point binding by ∼20% or more, ultrasensitivity is higher for the two-point model, even in the presence of substantial negative coupling (Supplementary Fig. 7d). Thus, the proposed ultrasensitivity enhancement effect for the two-site model is robust.
). Negative coupling is, however, required for a similar transition midpoint in the one- and two-site models (Supplementary Fig. 7c). Comparing ultrasensitivity for different degrees of negative coupling between the two Cdc4 binding pockets, we found that as long as the bound Sic1 conformations are held closer on average to the Cdc4 for two-point than for one-point binding by ∼20% or more, ultrasensitivity is higher for the two-point model, even in the presence of substantial negative coupling (Supplementary Fig. 7d). Thus, the proposed ultrasensitivity enhancement effect for the two-site model is robust.
Discussion
Multisite phosphorylation is often the basis for cellular decision-making37 and can control kinase priming events38, substrate dephosphorylation, subcellular localization and signal integration39. Multisite phosphorylation can lead to either linear graded responses40 or non-linear ultrasensitivity6,11,12. Sic1-Cdc4 is a well-studied example of an ultrasensitive interaction6,7,8,13,14. However, the molecular basis for Sic1 multisite-dependence has been elusive, in part because the consequences of substrate interactions on Cdc4 itself have never been investigated. Our assignment of 13 methyl groups positioned throughout the Cdc4 WD40 domain provides the first effective NMR probes for investigating substrate effects on Cdc4WD40. This approach enabled detection of unexpected direct binding interactions of Sic1 CPDs to the previously described SCF-I2 allosteric pocket, a finding with major ramifications. First, the allosteric pocket tethers Sic1 near the primary CPD pocket, curtailing Sic1 diffusion away from Cdc4 and increasing Sic1 local concentration. Second, based on affinity measurements, engagement of the allosteric pocket by CPDs weakens other CPD interactions with the primary pocket, likely by inducing rearrangement of P−2 sub-pocket residues analogous to the effect of SCF-I2. This negative allosteric effect facilitates the exchange of multiple CPD motifs, and increases the probability of rebinding another CPD site. The allosteric effect also explains the decreased chemical shift perturbations in the P−2 sub-pocket for extended multisite pSic1 substrates compared to short CycE and Sic1 phosphopeptides.
Identification of the allosteric pocket as a second direct binding determinant adds complexity to the already intricate nature of multisite Cdc4 substrate interactions. Since the sequence composition of individual CPD motifs varies considerably, some may bind to the allosteric pocket with higher affinity. Because of negative allosteric coupling between the primary CPD and allosteric pockets, CPDs that preferentially bind the allosteric pocket may have complex effects on substrate recognition. As the primary CPD and allosteric pockets are separated by ∼25 Å, simultaneous engagement can only occur if CPDs are separated by at least 7 residues. In Sic1, all but two CPD pairs meet this requirement, making many binding scenarios possible. These subtleties may affect timing of substrate degradation and confer differential susceptibility to small molecule allosteric inhibitors. We speculate that non-phosphorylated motifs in extended substrates may also engage the allosteric site to further refine substrate recognition.
Different models have been proposed for Sic1-Cdc4 ultrasensitivity. One initial proposal posited that a potential second binding pocket on Cdc4 could tether Sic1 to increase local concentration41. However, a simple two-site model would be dominated by overly strong thermodynamic effects, rendering high-affinity interaction dependent only on two sites, not the multiple sites observed. A kinetic model argued that, if internal CPD motion exceeded overall Sic1 diffusion, the probability of rebinding should exhibit an ultrasensitive dependence on number of CPDs42, but the physical basis for this effect was unknown. An entropic model postulated that multisite phosphorylation could significantly reduce conformational entropy for unbound Sic1 to favour binding43, but further experiments revealed no substantial decrease in Sic1 conformational disorder upon phosphorylation14.
Our polyelectrostatic model suggested that long-range interactions between the positively charged CPD pocket on Cdc4 and the cohort of negatively charged phosphates on Sic1 could yield an ultrasensitive transition once the overall Sic1 charge became negative35, analogous to other bulk electrostatic effects44. However, as many Sic1 lysines are dispensable for recognition45, charge balance alone may not explain ultrasensitivity. Regardless, it is necessary for all models that non-contacting CPDs contribute to binding such that the bound Sic1 population increases approximately exponentially with the number of phosphorylation events. Our modelling suggests that, if the allosteric pocket contributes significantly only for intermediate to large numbers of CPDs, then disabling the allosteric pocket will decrease the sharpness but not the midpoint of the ultrasensitive transition (Supplementary note 3). This predicted effect of an allosterically-coupled two-site interaction is generalizable to other mechanisms, including tethering41 and transient kinetic trapping42. To our knowledge, this form of negative allostery involving multiple biding sites within the same extended protein ligand has not been reported previously. Importantly, it has implications for the Sic1 conformational distribution that future, more comprehensive models will need to quantitatively consider (Supplementary note 4).
Allosteric communication between binding sites may be a general feature of WD40 domains, which frequently contact more than one partner on different surfaces, in some instances mediating allostery. In one example, the transducin inhibitor phosducin binds the transducin WD40 domain to induce structural rearrangements between blades 6 and 7, creating a binding pocket for the farnesyl moiety of the partner Gγ subunit27. Remarkably, the key allosteric pocket residues and main chain movements are virtually superimposable between transducin and Cdc4 (ref. 26). In another example, the transcriptional regulator WDR5 interacts with RbBP5 between blades 5 and 6 to allosterically regulate the methyltransferase activity of MLL1, which binds to an arginine-rich site on WDR5 (ref. 46). As shown here, binding of the same ligand to two different sites can also elicit allosteric communication and engender unusual protein recognition behaviour, vastly diversifying the output of simple binding events. Finally, we note that the Cdc4 allosteric infrastructure appears conserved in Fbw7 (ref. 26), which is a frequently mutated tumour suppressor in human cancer22. Since many Fbw7 substrates are multisite phosphorylated22, it will be of considerable interest to determine whether allosteric coupling influences Fbw7 substrate recognition, and whether substrate-selective Fbw7 allosteric inhibitors can be developed.
Methods
Protein production and purification
Cdc4WD40 (residues 367-744) was expressed from pET-Sumo (Invitrogen), isolated by Ni-nitrilotriacetate (NTA) affinity chromatography, digested with Ulp protease, and further purified by Ni-NTA and Superdex 200 chromatography8. Mutations to Cdc4WD40 in pETSumo vector were introduced using the Quickchange (Stratagene) approach and verified by DNA sequencing. For the NMR experiments, Escherichia coli BL21(DE3) codonPlus cells were transformed with pETSumo plasmid encoding Cdc4WD40 and were grown in 99% D2O minimal medium. To achieve methyl labelling of the Ile-δ1-[13CH3] and Met-ɛ-[13CH3] (referred to as IM-labelled Cdc4WD40 that is U-[15N, 2H], Ileδ1-[13CH3], Metɛ-[13CH3]) the media was supplemented with 50 mg l−1 alpha-ketobutyric acid ([methyl-13CHD2])47 and 100 mg l−1 L-methionine (methyl-13CH3)48,49 1 h before protein induction and was purified as described above8. Sic1 was expressed as a GST fusion protein in Escherichia coli BL21(DE3) codon plus cells. The GST-Sic1 protein was purified by using glutathione–Sepharose affinity chromatography. After digestion with TEV protease, GST was removed by using anion exchange chromatography. Sic1 was further purified to homogeneity by using a Superdex 75 column (Amersham Biosciences). Sic1 was then phosphorylated with immobilized Cln2-Cdc28 kinase from baculovirus-infected Sf9 cells13.
NMR spectroscopy
Assignment of Ile and Met residues in Cdc4WD40. All NMR experiments were performed at 10 °C on Varian Inova 500 and 800 MHz spectrometers. Methyl-TROSY spectra were recorded on per-deuterated protein samples dissolved in 100% D2O, 50 mM Na2HPO4, pH 8.0, 150 mM NaCl, 2 mM DTT. In order to assign the methyl residues, a total of 15 Ile to Val or Met to Val point mutations were made (I385V, I573V, M590V, I716V, I676V, I594V, I396V, I404V, I553V, I565V, I586V, I596V, I654V and I577V), which resulted in unambiguous assignment of 1 Met and 13 Ile residues. (I404V mutation led to a spectrum of very poor quality that could not be used for assignment.) All spectra were processed using the program NMRPipe50 and the spectra were analysed using SPARKY (T.D. Goddard and D.G. Kneller, SPARKY 3 (University of California, San Francisco).
NMR chemical shift perturbations. To monitor the interaction of Cdc4WD40 with different partners, 2D 13C–1H TROSY HMQC spectra were recorded at 10 °C on 200 μM IM-Cdc4WD40 sample for increasing concentrations of pSic1, CycE and Ash1 peptides and SCF-I2 inhibitor. Chemical shift changes that were greater than one standard deviation from the mean were considered significant. Chemical shift perturbations (CSPs) were calculated from the relations

where Δδ is the chemical shift change between the apo and the fully saturated states of the protein.
Methyl-utilizing cross-saturation experiment. The cross-saturation (CS) experiment was carried out as described in Takahashi et al.31 on a 300 μM IM-labelled Cdc4WD40 sample with 600 μM pSic in 100% D2O, 50 mM Na2HPO4, pH 8.0, 150 mM NaCl, 2 mM DTT buffer at 10 °C. Saturation of the aromatic protons in Sic1 was done by a WURST-2 adiabatic pulse with a maximum radiofrequency (RF) amplitude of 0.19 kHz. The saturation frequency was set to 7.0 with a saturation time of 1.5 s. As the saturation scheme was highly selective for the aromatic proton region, the irradiation did not affect the intensity of the pSic1 amide region. However, to ensure that CS effects seen were not transmitted by spin diffusion, a control experiment was performed in which the saturation frequency was set to −5 p.p.m. CS effects are presented as ratios of (Iirr/I0) with Cdc4 and (Iirr/I0) without Cdc4 [(Iirr/I0)Cdc4/(Iirr/I0)woCdc4].
Molecular docking
Models for Cdc4WD40-Ash1 and Cdc4WD40-Sic120pS69/pS76/pS80 complexes were obtained using the molecular docking program HADDOCK 2.1 (ref. 51), based on chemical shift perturbations for Cdc4WD40. Active and passive residues were selected based on the strategy outlined in ref. 33 and see also Supplementary Note 2. Docked structures, corresponding to the 200 best solutions with the lowest intermolecular energies, were generated and clustered based on a backbone RMSD tolerance of 1.0 Å. The quality of the structural ensemble was assessed using the PROCHECK program52.
Isothermal titration calorimetry (ITC)
ITC experiments were performed using VP-ITC and iTC200 microcalorimeters (MicroCal Inc., Northampton, MA) at 20 °C with the sample cell containing 30 μM Cdc4WD40 and the syringe loaded with 100 μM pSic1 or 200 μM SCF-I2. The samples were dialysed in buffer containing 50 mM Na2HPO4, pH 7.2, 150 mM NaCl, 2 mM β-mercaptoethanol. Runs were performed using 13–20 injections at an interval of 120 s and each binding experiment was repeated twice. Using the Origin software provided by MicroCal Inc., heat associated with each injection was integrated and thermodynamic parameters were extracted by fitting the data to a single-site binding model.
Yeast strains and cell culture
Wild type or mutant CDC4 (Arg655Gln, Arg664Gln, Arg655Gln/Arg664Gln) alleles under control of the native CDC4 promoter were introduced on a <CDC4 TRP CEN> plasmid into a cdc4ΔHIS3 strain in either W303 (MATa ade2-1 can1-100 his3-11,15 leu2-3, 112 trp1-1 ura3 GAL11 psi+) or S288C (BY4741 MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) backgrounds. Each strain was also co-transformed with a <GAL1-SIC1 LEU CEN> plasmid to allow conditional high level expression of SIC1. For microscopy and FACS analysis of DNA content, cells were grown to early log phase and induced overnight in selective galactose medium before imaging and staining with Sytox green dye8. For spot dilution assays, strains were cultured for one day in SC-Trp-Leu 2% raffinose liquid medium, then spotted in 10-fold dilutions onto SC-Trp-Leu 2% glucose or SC-Trp-Leu 2% raffinose 2% galactose plates and grown at 30 °C for three days.
Intrinsic Trp fluorescence measurements
The binding affinities of three different peptides (Sic120pS69, Sic120pS80 and Sic120pS69/S80) for wild-type and mutant (Arg664Gln) Cdc4WD40 were measured by intrinsic Trp fluorescence-binding experiments. The measurements were carried out by using an ATF 105 spectrometer (AVIV Instruments, Lakewood, NJ) equipped with an automatic titrator. The binding of the peptide fragments were monitored by using intrinsic tryptophan fluorescence with an excitation wavelength of 298 nm and an emission wavelength of 366 nm. All measurements were performed in 50 mM Tris, 150 mM NaCl, 5 mM DTT, pH 7.5 at 20 °C. The concentration of Cdc4 was 0.5 μM for all measurements35. Dissociation constants were determined by assuming 1:1 protein stoichiometry, given that the allosteric site interaction is not detectably perturbed by singly phosphorylated CPD peptides. Association constants were calculated using the formula Ka=1/Kd. Prism 4.0 software (GraphPad Software, San Diego, CA) was used for statistical analysis (non-paired Student t test), and P values <0.05 were considered significant. Each experiment was repeated at least three times.
Fluorescence polarization assay
Fluorescence polarization assays containing 0.21 μM Skp1-Cdc4 complex and 10 nM fluorescently labelled cyclin E-derived or Ash1-derived phosphopeptides in 10 mM HEPES (pH 7.5), 40 mM NaCl, 0.1 mg ml−1 BSA and 1 mM DTT in a final volume of 23.5 μl per well were performed on an Analyst HT plate reader (Molecular Devices Corp., Sunnyvale CA). Samples were excited at 485 nm and emission was read at 535 nm (ref. 26).
Mathematical model
We developed a variation of the mean-field electrostatic model35. Whereas the original model assumed only one Sic1 binding pocket on Cdc4, the model here embodies both a primary (P) and an allosteric (A) binding pocket. Accordingly, the Sic1-Cdc4 bound state consisted of three different bound configurations. To provide rationalization for the present experimental data, contact energies associated with the two binding pockets were chosen to represent experimentally observed trends. Additional energetic parameters that were likely unrealistic were also considered to explore alternate hypothetical binding scenarios and to assess the robustness of the proposed mechanism. Details are provided in the Supplementary Note 3.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
Additional information
How to cite this article: Csizmok, V. et al. An allosteric conduit facilitates dynamic multisite substrate recognition by the SCFCdc4 ubiquitin ligase. Nat. Commun. 8, 13943 doi: 10.1038/ncomms13943 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Stirnimann, C. U., Petsalaki, E., Russell, R. B. & Muller, C. W. WD40 proteins propel cellular networks. Trends Biochem. Sci. 35, 565–574 (2010).
Willems, A. R., Schwab, M. & Tyers, M. A hitchhiker's guide to the cullin ubiquitin ligases: SCF and its kin. Biochim. Biophys. Acta 1695, 133–170 (2004).
Petroski, M. D. & Deshaies, R. J. Function and regulation of cullin-RING ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 6, 9–20 (2005).
Skaar, J. R., Pagan, J. K. & Pagano, M. Mechanisms and function of substrate recruitment by F-box proteins. Nat. Rev. Mol. Cell Biol. 14, 369–381 (2013).
Verma, R. et al. Phosphorylation of Sic1p by G1 Cdk required for its degradation and entry into S phase. Science 278, 455–460 (1997).
Nash, P. et al. Multisite phosphorylation of a CDK inhibitor sets a threshold for the onset of DNA replication. Nature 414, 514–521 (2001).
Orlicky, S., Tang, X., Willems, A., Tyers, M. & Sicheri, F. Structural basis for phosphodependent substrate selection and orientation by the SCFCdc4 ubiquitin ligase. Cell 112, 243–256 (2003).
Tang, X. et al. Composite low affinity interactions dictate recognition of the cyclin-dependent kinase inhibitor Sic1 by the SCFCdc4 ubiquitin ligase. Proc. Natl Acad. Sci. USA 109, 3287–3292 (2012).
Serber, Z. & Ferrell, J. E. Jr Tuning bulk electrostatics to regulate protein function. Cell 128, 441–444 (2007).
Ha, S. H. & Ferrell, J. E. Jr Thresholds and ultrasensitivity from negative cooperativity. Science 352, 990–993 (2016).
Ferrell, J. E. Jr Tripping the switch fantastic: how a protein kinase cascade can convert graded inputs into switch-like outputs. Trends Biochem. Sci. 21, 460–466 (1996).
Salazar, C. & Hofer, T. Allosteric regulation of the transcription factor NFAT1 by multiple phosphorylation sites: a mathematical analysis. J. Mol. Biol. 327, 31–45 (2003).
Mittag, T. et al. Dynamic equilibrium engagement of a polyvalent ligand with a single-site receptor. Proc. Natl Acad. Sci. USA 105, 17772–17777 (2008).
Mittag, T. et al. Structure/function implications in a dynamic complex of the intrinsically disordered Sic1 with the Cdc4 subunit of an SCF ubiquitin ligase. Structure 18, 494–506 (2010).
Hao, B., Oehlmann, S., Sowa, M. E., Harper, J. W. & Pavletich, N. P. Structure of a Fbw7-Skp1-cyclin E complex: multisite-phosphorylated substrate recognition by SCF ubiquitin ligases. Mol. Cell 26, 131–143 (2007).
Koivomagi, M. et al. Cascades of multisite phosphorylation control Sic1 destruction at the onset of S phase. Nature 480, 128–131 (2011).
Welcker, M. & Clurman, B. E. Fbw7/hCDC4 dimerization regulates its substrate interactions. Cell Div. 2, 7 (2007).
Welcker, M. et al. Fbw7 dimerization determines the specificity and robustness of substrate degradation. Genes Dev. 27, 2531–2536 (2013).
Tang, X. et al. Suprafacial orientation of the SCFCdc4 dimer accommodates multiple geometries for substrate ubiquitination. Cell 129, 1165–1176 (2007).
Liu, Q. et al. SCFCdc4 enables mating type switching in yeast by cyclin-dependent kinase-mediated elimination of the Ash1 transcriptional repressor. Mol. Cell Biol. 31, 584–598 (2011).
Duda, D. M. et al. Structural regulation of cullin-RING ubiquitin ligase complexes. Curr. Opin. Struct. Biol. 21, 257–264 (2011).
Davis, R. J., Welcker, M. & Clurman, B. E. Tumor suppression by the Fbw7 ubiquitin ligase: mechanisms and opportunities. Cancer Cell 26, 455–464 (2014).
Smock, R. G. & Gierasch, L. M. Sending signals dynamically. Science 324, 198–203 (2009).
Gunasekaran, K., Ma, B. & Nussinov, R. Is allostery an intrinsic property of all dynamic proteins? Proteins 57, 433–443 (2004).
Hilser, V. J. Biochemistry. An ensemble view of allostery. Science 327, 653–654 (2010).
Orlicky, S. et al. An allosteric inhibitor of substrate recognition by the SCF(Cdc4) ubiquitin ligase. Nat. Biotechnol. 28, 733–737 (2010).
Loew, A., Ho, Y. K., Blundell, T. & Bax, B. Phosducin induces a structural change in transducin beta gamma. Structure 6, 1007–1019 (1998).
Verma, R., Feldman, R. M. & Deshaies, R. J. SIC1 is ubiquitinated in vitro by a pathway that requires CDC4, CDC34, and cyclin/CDK activities. Mol. Biol. Cell 8, 1427–1437 (1997).
Rosenzweig, R. & Kay, L. E. Bringing dynamic molecular machines into focus by methyl-TROSY NMR. Annu. Rev. Biochem. 83, 291–315 (2014).
Tugarinov, V., Hwang, P. M., Ollerenshaw, J. E. & Kay, L. E. Cross-correlated relaxation enhanced 1H-13C NMR spectroscopy of methyl groups in very high molecular weight proteins and protein complexes. J. Am. Chem. Soc. 125, 10420–10428 (2003).
Takahashi, H. et al. Utilization of methyl proton resonances in cross-saturation measurement for determining the interfaces of large protein-protein complexes. J. Biomol. NMR 34, 167–177 (2006).
Zhou, H. X. Quantitative account of the enhanced affinity of two linked scFvs specific for different epitopes on the same antigen. J. Mol. Biol. 329, 1–8 (2003).
Dominguez, C., Boelens, R. & Bonvin, A. M. HADDOCK: a protein-protein docking approach based on biochemical or biophysical information. J. Am. Chem. Soc. 125, 1731–1737 (2003).
Vauquelin, G. & Charlton, S. J. Exploring avidity: understanding the potential gains in functional affinity and target residence time of bivalent and heterobivalent ligands. Br. J. Pharmacol. 168, 1771–1785 (2013).
Borg, M. et al. Polyelectrostatic interactions of disordered ligands suggest a physical basis for ultrasensitivity. Proc. Natl Acad. Sci. USA 104, 9650–9655 (2007).
Song, J., Ng, S. C., Tompa, P., Lee, K. A. W. & Chan, H. S. Polycation-pi interactions are a driving force for molecular recognition by an intrinsically disordered oncoprotein family. PLoS Comput. Biol. 9, e1003239 (2013).
Cohen, P. The regulation of protein function by multisite phosphorylation--a 25 year update. Trends Biochem. Sci. 25, 596–601 (2000).
McGrath, D. A. et al. Cks confers specificity to phosphorylation-dependent CDK signaling pathways. Nat. Struct. Mol. Biol. 20, 1407–1414 (2013).
Schweiger, R. & Linial, M. Cooperativity within proximal phosphorylation sites is revealed from large-scale proteomics data. Biol. Direct. 5, 6 (2010).
Holmberg, C. I., Tran, S. E., Eriksson, J. E. & Sistonen, L. Multisite phosphorylation provides sophisticated regulation of transcription factors. Trends Biochem. Sci. 27, 619–627 (2002).
Deshaies, R. J. & Ferrell, J. E. Jr Multisite phosphorylation and the countdown to S phase. Cell 107, 819–822 (2001).
Klein, P., Pawson, T. & Tyers, M. Mathematical modeling suggests cooperative interactions between a disordered polyvalent ligand and a single receptor site. Curr. Biol. 13, 1669–1678 (2003).
Lenz, P. & Swain, P. S. An entropic mechanism to generate highly cooperative and specific binding from protein phosphorylations. Curr. Biol. 16, 2150–2155 (2006).
Strickfaden, S. C. et al. A mechanism for cell-cycle regulation of MAP kinase signaling in a yeast differentiation pathway. Cell 128, 519–531 (2007).
Petroski, M. D. & Deshaies, R. J. Context of multiubiquitin chain attachment influences the rate of Sic1 degradation. Mol. Cell 11, 1435–1444 (2003).
Avdic, V. et al. Structural and biochemical insights into MLL1 core complex assembly. Structure 19, 101–108 (2011).
Goto, N. K., Gardner, K. H., Mueller, G. A., Willis, R. C. & Kay, L. E. A robust and cost-effective method for the production of Val, Leu, Ile (δ 1) methyl-protonated 15N-, 13C-, 2H-labeled proteins. J. Biomol. NMR 13, 369–374 (1999).
Gelis, I. et al. Structural basis for signal-sequence recognition by the translocase motor SecA as determined by NMR. Cell 131, 756–769 (2007).
Religa, T. L., Sprangers, R. & Kay, L. E. Dynamic regulation of archaeal proteasome gate opening as studied by TROSY NMR. Science 328, 98–102 (2010).
Delaglio, F. et al. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 6, 277–293 (1995).
de Vries, S. J., van Dijk, M. & Bonvin, A. M. The HADDOCK web server for data-driven biomolecular docking. Nat. Protoc. 5, 883–897 (2010).
Laskowski, R. A., MacArthur, M. W., Moss, D. S. & Thornton, J. M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Cryst. 26, 283–291 (1993).
Acknowledgements
We thank Ranjith Muhandiram and Lewis Kay for assistance with NMR experiments and Debbie Blake for assistance with yeast experiments. This work was supported by grants from the Canadian Institutes of Health Research (MOP-57795, MOP-84281, MOP-126129 and MOP-114985 to F.S., H.S.C., M.T. and J.D.F.-K, respectively) and the Canadian Cancer Society Research Institute (703906 to F.S. and M.T., 703477 to H.S.C. and J.D.F.-K.), by an award from the Ministère de l’enseignement supérieur, de la recherche, de la science et de la technologie du Québec through Génome Québec to M.T. by a Canada Research Chair in Structural Biology to F.S., by a Canada Research Chair in Systems and Synthetic Biology to M.T. and by a Canada Research Chair in Intrinsically Disordered Proteins to J.D.F.-K.
Author information
Authors and Affiliations
Contributions
V.C., S.O., J.C., N.D., J.S., H.S.C., M.T., T.M. and J.D.F.-K. designed the experiments. V.C., S.O., J.C., A.B., H.L., N.D. and J.S. performed the experiments. V.C., S.O., J.C., J.S., H.S.C., F.S., M.T. and J.D.F.-K. contributed to the data analysis. V.C., T.M., H.S.C., F.S., M.T. and J.D.F.-K. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures, Supplementary Notes and Supplementary References (PDF 1679 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Csizmok, V., Orlicky, S., Cheng, J. et al. An allosteric conduit facilitates dynamic multisite substrate recognition by the SCFCdc4 ubiquitin ligase. Nat Commun 8, 13943 (2017). https://doi.org/10.1038/ncomms13943
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms13943
This article is cited by
-
Flexibility of intrinsically disordered degrons in AUX/IAA proteins reinforces auxin co-receptor assemblies
Nature Communications (2020)
-
Surface charge of Merkel cell polyomavirus small T antigen determines cell transformation through allosteric FBW7 WD40 domain targeting
Oncogenesis (2020)
-
WD40 repeat domain proteins: a novel target class?
Nature Reviews Drug Discovery (2017)
-
A hidden competitive advantage of disorder
Nature (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.