Abstract
Widespread application of thermoelectric devices for waste heat recovery requires low-cost high-performance materials. The currently available n-type thermoelectric materials are limited either by their low efficiencies or by being based on expensive, scarce or toxic elements. Here we report a low-cost n-type material, Te-doped Mg3Sb1.5Bi0.5, that exhibits a very high figure of merit zT ranging from 0.56 to 1.65 at 300−725 K. Using combined theoretical prediction and experimental validation, we show that the high thermoelectric performance originates from the significantly enhanced power factor because of the multi-valley band behaviour dominated by a unique near-edge conduction band with a sixfold valley degeneracy. This makes Te-doped Mg3Sb1.5Bi0.5 a promising candidate for the low- and intermediate-temperature thermoelectric applications.
Similar content being viewed by others
Introduction
Thermoelectric conversion technology, which can realize the direct and reversible conversion between heat and electricity without moving parts, offers a promising solution to critical energy and environmental challenges1. The conversion efficiency of thermoelectric devices is determined by the materials performance that is quantified by the dimensionless figure of merit, zT=α2σT/κ, where α is the Seebeck coefficient, σ is the electrical conductivity, T is the absolute temperature and κ is the thermal conductivity. Numerous efforts worldwide have been directed towards the improvement of zT through enhancing the power factor2,3,4,5,6 (α2σ) and reducing the thermal conductivity7,8,9,10.
Widespread application of thermoelectric technology calls for low-cost, environmentally benign and nontoxic high-performance thermoelectric materials. Current commercial materials contain a large amount of the rare element tellurium or the toxic element lead (for example, Bi2Te3 or PbTe) that prohibits large-scale applications. Zintl compounds such as skutterudites11, clathrates12 and zinc antimonides13 are a promising class of thermoelectric materials with high figure of merit. However, these materials often contain expensive, scarce or toxic heavy elements such as Ge, Co, Yb, Eu and Cd. Among them, Mg3Sb2-based Zintl compounds are a potential source of environmentally friendly, earth-abundant and inexpensive materials. The earth abundance of magnesium is several orders of magnitude larger than that of other elements like Zn, Co, Ga and Ge in the earth’s crust. Mg3Sb2 was initially investigated with the hope of realizing high zT value for the intermediate- and high-temperature applications14. However, the thermoelectric performance of Mg3Sb2 is rather low and severely limited by poor electrical transport properties, even though it has reasonably low thermal conductivity. Many strategies, including theoretical orbital engineering4 and experimental tuning of hole carrier concentration via various dopants15,16,17,18, have been developed for optimizing p-type performance, whereas nearly no attempt19 has been made for n-type doping in these compounds. In fact, all known Zintl antimonides except skutterudites have been reported to be p-type, and this appears to be because of intrinsic point defects20. It is a great challenge to achieve n-type properties in Mg3Sb2-based Zintl compounds.
Using combined theoretical prediction and experimental validation, here we present successful n-type doping in Mg3Sb1.5Bi0.5 solid solution using tellurium and obtain a strongly enhanced zT over a wide range of temperatures, from 0.56 to 1.65 at 300−725 K in Mg3Sb1.48Bi0.48Te0.04, compared with p-type undoped Mg3Sb2 (ref. 16) with zT from 0.002 to 0.26 at 332−750 K (Fig. 1a). The thermoelectric performance of n-type Mg3Sb1.5−0.5xBi0.5−0.5xTex (x=0.04, 0.05 and 0.08) is at least 2 times higher than that of p-type Mg3Sb2-based compounds including Na-doped Mg3Sb2 (ref. 18) and Mg3Sb1.8Bi0.2 (ref. 16) throughout the whole temperature range, outperforming other p-type CaAl2Si2-type Zintl compounds21,22 including EuZn1.8Cd0.2Sb2 and YbCd1.6Zn0.4Sb2 that have the highest reported thermoelectric performance so far. Moreover, high n-type performance at 300−725 K shown in Mg3Sb1.48Bi0.48Te0.04 is comparable to the best current state-of-the-art n-type materials5,12,23,24,25,26 such as Bi2Te3−xSex and AgPbmSbTe2+m (Fig. 1b) that, however, contain a large amount of expensive, scarce or toxic heavy elements such as Te, Ag and Pb. Combining theory and experiment, we show that the exceptionally high thermoelectric performance originates from a considerably enhanced power factor that is aided by the combination of a low resistivity and an enhanced Seebeck coefficient contributed by the multiple band behaviour dominated by a unique near-edge conduction band with a high valley degeneracy of 6.
(a) zT values of n-type Mg3Sb1.5−0.5xBi0.5−0.5xTex (x=0.04, 0.05, 0.08 and 0.20) in comparison with p-type undoped Mg3Sb2 (ref. 16), Na-doped Mg3Sb2 (ref. 18), Mg3Sb1.8Bi0.2 (ref. 16), EuZn1.8Cd0.2Sb2 (ref. 22) and YbCd1.6Zn0.4Sb2 (ref. 21). (b) zT comparison of n-type Mg3Sb1.48Bi0.48Te0.04 and current state-of-the-art n-type thermoelectric materials, Cu-doped Bi2Te3−xSex (ref. 23), AgPbmSbTe2+m (ref. 24), Mg2Si-Mg2Sn (ref. 5), Ba0.08La0.05Yb0.04Co4Sb12 (ref. 25), PbTe-Ag2Te-La (ref. 26) and Ba8Ga16Ge30 (ref. 12).
Results
Multi-valley conduction bands in n-type Mg3Sb2
The Mg3Sb2 Zintl compound with a crystal structure similar to CaAl2Si2 is intrinsically p-type that might be attributed to the point defects at Mg sites20. Many efforts have been directed towards p-type doping using a variety of dopants, whereas nearly no attempt has been made for n-type doping. The present work is intuitively focussed on searching for potential high performance n-type thermoelectric candidates from Zintl compounds. By combining full band structure calculations and semiclassical Boltzmann transport theory, the Seebeck coefficient and power factor of both n-type and p-type Mg3Sb2 are estimated and shown in Fig. 2a,b. We find that the Seebeck coefficient and power factor of n-type doping are much better than those of p-type doping for binary Mg3Sb2. Interestingly, the n-type electrical transport properties are found to be related to a unique conduction band with 6 conducting carrier pockets along the M–L line (Fig. 2c,d).
(a) The magnitude of Seebeck coefficient (|α|) versus Hall carrier concentration (nH) at 300 K. (b) Power factor as a function of Hall carrier concentration. In (a,b), the black and red solid lines represent the prediction of p-type and n-type Mg3Sb2 from full density functional theory (DFT) band structure calculation. Green and purple dashed lines show the expected |α| versus nH behaviour for single parabolic bands with effective masses equal to the two individual conduction bands ML and K at the band edges. The black solid points are the reported p-type doped Mg3Sb2 from the refs 17, 18. The pink star points represent n-type Te-doped Mg3Sb1.5Bi0.5 Zintl compounds from our own work. Our experimental data lie below the curve by DFT for Mg3Sb2 because there is an energy difference of 0.12 eV between the ML band and K band in n-type Mg3Sb1.5Bi0.5, whereas these two bands are nearly converged in Mg3Sb2 (see Fig. 3a). (c) Calculated electronic band structure and partial density of states (DOS) for Mg3Sb2. (d,e) Calculated Fermi surfaces of (d) n-type and (e) p-type Mg3Sb2 at the Fermi level 0.03 eV above conduction band minimum and 0.1 eV below valence band maximum, respectively. The front sides of Fermi surfaces are plotted in yellow, whereas the back sides are coloured in blue. Black dots represent the high-symmetry k-points. The high-symmetry M-L line is marked in red colour. Fermi surface of n-type Mg3Sb2 exhibits 6 anisotropic carrier pockets along the M-L line and 6 one-third pockets at the K point, whereas p-type Mg3Sb2 possesses only one highly anisotropic carrier pocket at the Γ point.
Several methods shown in Fig. 2 are used to elucidate the multiple band characteristics in n-type Mg3Sb2, responsible for the exceptional electrical transport performance. Ab initio band structure calculation by density functional theory (DFT) shows an indirect band gap of 0.6 eV in Mg3Sb2 with a valence band maximum located at the Γ point and a conduction band minimum at the K point (Fig. 2c). The conduction band at the K point (the K band) in the Brillouin zone possesses a valley degeneracy of 2. However, there is a secondary conduction band (the ML band) located just above the K band along the M–L high symmetry line and this ML band shows a valley degeneracy of 6. The calculation predicts a rather small energy difference of 0.02 eV between these two conduction bands. Such a small energy difference, comparable to kBT at room temperature, implies that the two conduction bands are nearly converged. The ML band covers an energy range of 0.19 eV at the band edge and in this energy window a steep increase of density of states (DOS) appears (Fig. 2c). Moreover, there are two other conduction bands (the M band and L band) located at 0.12 and 0.21 eV above the K band. In contrast to the multi-valley bands at the conduction band minimum, there is only one near-edge valence band at the Γ point (the Γ band). As a result of the large DOS induced by multiple conduction bands, the Fermi level slowly moves up the conduction bands with increasing n-type doping concentration. At room temperature, the ML band can be easily reached as the electron doping concentration approaches ∼4 × 1019 cm−3, whereas approaching the M or L band minimum will require a doping level as high as ∼2 × 1020 or ∼6 × 1020 cm−3, respectively.
The multi-valley band feature can be directly observed by the iso-energy Fermi surface (see Fig. 2d). The iso-energy Fermi surface of n-type Mg3Sb2 for an energy level 0.03 eV above conduction band minimum shows 6 isolated full electron pockets along the M–L line inside the Brillouin zone and 6 one-third pockets at the K point. Accordingly, the valley degeneracies of the ML band and the K band are 6 and 2, respectively, that add up to 8. Such a high valley degeneracy is comparable to many widely used thermoelectric materials such as (Bi, Sb)2Te3 (ref. 27). In contrast, the Fermi surface of p-type Mg3Sb2 only shows one hole pocket at the Γ point (Fig. 2e). Given the above result, it is obvious that n-type doping will display much higher degeneracy of carrier pockets than that of p-type doping in Mg3Sb2.
Another clear illustration of the complex band behaviour can be seen in the doping-dependent Seebeck coefficient. The doping dependence of the Seebeck coefficient at 300 K shown in Fig. 2a is simulated using full band structure calculations as well as a single parabolic band model. The experimental data of p-type doped Mg3Sb2 agree very well with the curve simulated by integrated ab initio full band structure and semiclassical Boltzmann transport theory under a rigid band approximation, proving the effectiveness of this approach. The Seebeck coefficient is proportional to the DOS effective mass27,28, given by md*=Nv2/3ms*, where Nv represents the valley degeneracy and ms* is the single valley effective mass. The effective masses ms* of the K band and the ML band are respectively 0.28me and 0.32me, indicating light mass behaviours. However, owing to high valley degeneracy (Nv=6) the DOS effective mass of the ML band md*=1.05me is much heavier than that of the K band (md*=0.45me, Nv=2). The Seebeck coefficient estimated by a single band model with DOS effective mass of 0.45me from the K band is only −105 μV K−1 at 2.2 × 1019 cm−3, much smaller than full band structure calculated Seebeck coefficient of −230 μV K−1 at the same carrier concentration. Even using a single band model with the heavy mass from the ML band, the Seebeck coefficient (−190 μV K−1) at the same carrier concentration gives a value that is still lower than that of multiband DFT model. Therefore, a single parabolic band model cannot reproduce the Seebeck coefficient from full DFT band structure calculation, confirming the multiple band effects in n-type Mg3Sb2. Although the effective mass ms*=0.58me of the Γ band at the valence band maximum is relatively heavier than those of the K band and ML band, the low valley degeneracy (Nv=1) leads to a DOS effective mass of 0.58me of the Γ valence band, much smaller than that of the ML band. As expected, the Seebeck coefficient of p-type Mg3Sb2 predicted by full DFT calculation is lower than that of the single band model estimated by the ML band effective mass. The above results reveal that the ML band with a high valley degeneracy of 6 makes an important contribution to the high Seebeck values.
The optimal electrical transport performance is determined by the weighted mobility, μ(md*/me)3/2, where μ is the carrier mobility and me is the mass of an electron3,28. Taking the assumption of acoustic phonon scattering for charge carriers, the carrier mobility can be expressed as μ∝1/ms*5/2 (ref. 27). Considering the expression of the DOS effective mass, the weighted mobility can be simplified and expressed as proportional to Nv/ms*. Thus, the ML band with a sixfold valley degeneracy and light effective mass is highly desirable for the electrical performance. The high valley degeneracy Nv=6 has the effect of producing a large DOS effective mass and thereby a high Seebeck coefficient without explicitly reducing the mobility. In addition, compared with the Γ valence band, the light effective mass ms* of the ML band is beneficial to increase μ and thus improve electrical conductivity σ. Therefore, it is clear that the ML band plays a crucial role in the strongly enhanced power factor shown in Fig. 2b.
Exploring potential candidates with multi-valley conduction bands
In order to understand whether the conduction band minimum along the M–L line is unique in Mg3Sb2, we conduct a screening of the band structures from a variety of CaAl2Si2-type Zintl compounds using our previous computational methods4. As a result, we find that the conduction band minimum along the M–L line only exists in binary Mg3X2 (X=As, Sb, Bi), shown in Fig. 2c and Supplementary Fig. 1. The band gaps decrease as X goes down the periodic table from As (1.6 eV) to Sb (0.6 eV) to Bi (semimetal). The conduction bands at the band edges of these compounds depict very similar multiple band behaviours at the K point and along the M–L line. This is because the near-edge conduction bands are dominated by the electronic states of Mg, whereas the valence bands are contributed by Sb (Fig. 2c). Hence, doping at anion sites will only have a minor effect on the conduction band minimum and thereby will not destroy the multi-valley conduction bands. Therefore, we can expect that n-type doped Mg3Sb2 and Mg3As2 as well as solid solutions Mg3Sb2−xBix, Mg3Sb2−xAsx and Mg3As2−xBix with suitable band gaps will show enhanced power factor as well as thermoelectric performance if properly doped at the anion sites.
Multiple conduction band behaviour in n-type Mg3Sb1.5Bi0.5
To confirm the existence of multiple conduction bands and especially the ML band in the solid solutions, the electronic structures of Mg3Sb2−xBix solid solutions are simulated by DFT and the band structure of an exemplified solid solution Mg3Sb1.5Bi0.5 is shown in Fig. 3a. The result proves that multiple band behaviour including the ML band with a sixfold valley degeneracy is indeed preserved in Mg3Sb1.5Bi0.5 solid solution and the dispersions and effective masses of the K band and ML band are very similar to those of Mg3Sb2. The main difference, however, is that the ML band in Mg3Sb1.5Bi0.5 is shifted downward 0.12 eV below the K band and therefore becomes the conduction band minimum. This is good for the thermoelectric performance of Mg3Sb1.5Bi0.5 as the ML band with a high valley degeneracy of 6 can be easily reached with a relatively low doping level. Thus, it is of interest to understand the correlation between the composition of Mg3Sb2−xBix solid solutions and the energy difference of the K band and ML band, defined as ΔEK−ML=EK−EML. The dependence of ΔEK−ML on the fraction x is shown in Fig. 3b. Surprisingly, ΔEK−ML can be continuously tuned by the fraction x from −0.02 to 0.18 eV. Energy gaps of Mg3Sb2−xBix show a decreasing trend from 0.43 to 0.08 eV as x increases from 0.5 to 1.5 (Fig. 3b), suggesting that the bipolar effect will be obvious when x>1. The obvious bipolar effect for Mg3Sb2−xBix (x>1) is confirmed in the previous experimental report17. Hence, n-type Mg3Sb2−xBix (x≤1) compounds are very promising thermoelectric candidates if properly doped on the anion sites.
(a) Effective band structure of Mg3Sb1.5Bi0.5 solid solution. The spectral weight is represented by colour and the symbol size. Points with the spectral weight of <0.25 are filtered out. The band structure of Mg3Sb1.5Bi0.5 depicts a multiple conduction band behaviour similar to that of Mg3Sb2 (Fig. 2c), where the ML band possesses a sixfold valley degeneracy and the K band has a twofold valley degeneracy. However, the ML band in Mg3Sb1.5Bi0.5 becomes the conduction band minimum that is ∼0.12 eV below the K band. (b) The energy difference ΔEK−ML between the K band and the ML band and the band gap Eg as a function of the fraction x in Mg3Sb2−xBix solid solutions. Mg3Bi2 (x=2.0) is a semimetal. The solid lines represent fitted curves using a B spline. (c) Seebeck coefficient versus Hall carrier concentration at 725 K. The red solid line represents the prediction of n-type Mg3Sb2 from full density functional theory (DFT) band structure calculation. The green dashed line depicts the expected α versus nH behaviour at 725 K for a single parabolic band with an effective mass equal to the ML conduction band. The pink star points are the data of Te-doped Mg3Sb1.5Bi0.5 of this work. (d) Temperature dependence of the experimental DOS effective mass calculated from a single band model. The solid lines represent fitted curves using a B spline.
Achieving n-type Mg3Sb2 by doping tellurium on the anion sites has been attempted and found to be difficult, whereas it is easier in Mg3Sb2−xBix solid solutions. This is probably because of the lower formation energy of tellurium doping on the anion sites of Mg3Sb2−xBix solid solutions (see one example in Supplementary Fig. 2). From the above theoretical calculation, Mg3Sb1.5Bi0.5 possesses a small band gap of 0.43 eV as well as ΔEK−ML=0.12 eV with the ML band as the conduction band minimum (Fig. 3a,b), making it a potential candidate for n-type doping. The experimental validation is successfully demonstrated in n-type doped Mg3Sb1.5Bi0.5 solid solution using tellurium as an effective dopant. Mg3Sb1.5−0.5xBi0.5−0.5xTex (x=0.04, 0.05, 0.08 and 0.20) samples were synthesized by combining arc-melting process and spark plasma sintering (Supplementary Figs 3–5). The room temperature carrier concentration data of these samples show a small distribution from 1.18 × 1019 (x=0.20) to 2.20 × 1019 cm−3 (x=0.04) (see Supplementary Fig. 6a) and the corresponding doping levels are deep enough to cut the ML band.
The experimental Seebeck coefficients of n-type Mg3Sb1.5−0.5xBi0.5−0.5xTex samples at 300 or 725 K are larger than the Seebeck value calculated by a single band model using the DOS effective mass of the ML band (see Figs 2a and 3c). This result not only confirms the theoretical calculation that the ML band dominates the conduction band minimum of Mg3Sb1.5Bi0.5, but also reveals that the K band located at 0.12 eV above the conduction band minimum makes a contribution to both the room-temperature and high-temperature electrical transports. As there is an energy difference of 0.12 eV between the ML band and the K band in n-type Mg3Sb1.5Bi0.5, the experimental Seebeck coefficients of n-type Mg3Sb1.5−0.5xBi0.5−0.5xTex are smaller than the calculated value by DFT for n-type Mg3Sb2 with the effective convergence of the two bands (see Figs 2c and 3a,b).
The carrier concentration of Mg3Sb1.5−0.5xBi0.5−0.5xTex increases with increasing temperature and reaches 4.83 × 1019 cm−3 at 725 K in the high-performance sample with x=0.04 (Supplementary Fig. 6a), suggesting that the Fermi level will move upward approaching the K band with rising temperature. In addition, the broadening of the Fermi distribution makes the Fermi level easier to reach the K band at high temperatures. The temperature-dependent DOS effective masses in Te-doped Mg3Sb1.5Bi0.5 are illustrated in Fig. 3d. As shown in Fig. 3d, the DOS effective mass of Mg3Sb1.5−0.5xBi0.5−0.5xTex (x=0.04 and 0.05) derived from the experimental Seebeck coefficient increases with increasing temperature at 400–725 K, ruling out the single band behaviour at high temperatures. The above results again prove that the multiple band behaviour, including the effects from both the ML band and the K band, makes a contribution to the high-temperature transport properties.
Thermoelectric properties of n-type Te-doped Mg3Sb1.5Bi0.5
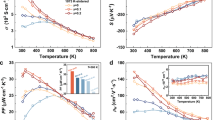
Figure 4a shows the temperature-dependent power factors of n-type Mg3Sb1.5−0.5xBi0.5−0.5xTex (x=0.04, 0.05, 0.08 and 0.20) samples. Among the n-type samples, Mg3Sb1.48Bi0.48Te0.04 has the highest power factor within the entire measurement temperature range. At room temperature, Mg3Sb1.48Bi0.48Te0.04 sample shows α2σ=13.9 μW cm−1 K−2 that reaches a maximum value of 14.14 μW cm−1 K−2 at 525 K and then decreases. Compared with other state-of-the-art thermoelectric materials, the power factors of n-type Mg3Sb1.5−0.5xBi0.5−0.5xTex samples are moderate, but much better than those of p-type Mg3Sb2-based materials (Mg3Sb2, Na-doped Mg3Sb2 and Mg3Sb1.8Bi0.2). We attribute the significant enhancements of the power factors in Te-doped Mg3Sb1.5Bi0.5 samples to the combination of the enhanced Seebeck coefficients and low resistivity values. The enhanced Seebeck coefficients are clearly demonstrated in the doping dependence of the Seebeck coefficients (Fig. 2a) as discussed above. In addition, the Seebeck coefficients of all Te-doped samples show increasing trends with increasing temperature. Typically, the Mg3Sb1.5−0.5xBi0.5−0.5xTex sample with x=0.04 exhibits the lowest absolute value of Seebeck coefficient that increases from 207 μV K−1 at 300 K to 268 μV K−1 at 725 K (Fig. 4b), higher than that of Na-doped Mg3Sb2 (ref. 18) at a comparable carrier concentration of 3.87 × 1019 cm−3. The room temperature Seebeck coefficient is comparable to other state-of-the-art thermoelectric materials such as 1% Cu-doped Bi2Te3−xSex (ref. 23) with α=−188 μV K−1 at a similar carrier concentration.
Temperature dependence of (a) power factor α2σ, (b) absolute values of Seebeck coefficient α, (c) electrical resistivity ρ and (d) total thermal conductivity κ of Mg3Sb1.5−0.5xBi0.5−0.5xTex (x=0.04, 0.05, 0.08 and 0.20) and the comparison with p-type undoped Mg3Sb2 (ref. 16), Na0.006Mg2.994Sb2 (ref. 18) and Mg3Sb1.8Bi0.2 (ref. 16).
The resistivity at room temperature shown in Fig. 4c decreases from 545 mΩ cm in undoped Mg3Sb2 to 3.09 mΩ cm in Mg3Sb1.48Bi0.48Te0.04, changing the temperature dependence from a semiconductor behaviour to a metallic behaviour. For the Te-doped samples, the resistivity ρ increases with increasing Te content, showing a similar trend as the Seebeck coefficient. For the Mg3Sb1.48Bi0.48Te0.04 sample with the highest power factor, ρ increases from 3.09 mΩ cm at 300 K to 5.67 mΩ cm at 725 K (Fig. 4c). The resistivity values of Mg3Sb1.5−0.5xBi0.5−0.5xTex (x=0.04, 0.05 and 0.08) are lower than that of Na-doped Mg3Sb2 (ref. 18) at a comparable carrier concentration (3.87 × 1019 cm−3). The low resistivity values of these samples at room temperature come from high mobility data of 62.4–91.8 cm2 V−1 s−1 (Supplementary Fig. 6b), a factor of at least 3.7 larger than the mobility17,18 of the undoped Mg3Sb2 (μ=16 cm2 V−1 s−1 at nH=1.3 × 1019 cm−3) and Na-doped Mg3Sb2 (μ=16.7 cm2 V−1 s−1 at nH=3.87 × 1019 cm−3). This reveals the light mass conduction band at the band edge induced by the ML band compared with the Γ valence band, consistent with the theoretical calculation. In addition, the carrier mobility data of n-type Te-doped samples with x=0.04, 0.05 and 0.08 roughly follow the temperature-dependent relation μ∝T−P (1≤P≤1.5) (Supplementary Fig. 6b), indicating that the electrical transport is dominated by acoustic phonon scattering. Using the mobility of the undoped Mg3Sb2 and ms* of the ML band (0.32me) and the Γ band (0.58me), the mobility of n-type Mg3Sb1.5Bi0.5 can be roughly estimated by μ∝1/ms*5/2 relation to be ∼71 cm2 V−1 s−1, comparable to the experimental values of the samples with x=0.04, 0.05 and 0.08. The above results thus confirm that the light mass of the ML band in Te-doped Mg3Sb1.5Bi0.5 indeed results in high mobility and thereby low resistivity that is favourable to the electrical transport.
The total thermal conductivity values of n-type Mg3Sb1.5−0.5xBi0.5−0.5xTex (x=0.04, 0.05, 0.08 and 0.20) samples are low and exhibit decreasing trends with increasing temperature (Fig. 4d). The lowest room-temperature thermal conductivity of 0.743 W m−1 K−1 is observed for the sample with x=0.04. The total thermal conductivity, κ, value decreases to 0.556 W m−1 K−1 at 725 K. Compared with p-type Mg3Sb2 and Mg3Sb1.8Bi0.2, the observed reduction in κ of Te-doped Mg3Sb1.5Bi0.5 is expected from the phonon scattering of alloys. It means that the thermal conductivity definitely also makes a significant contribution to high zT at low temperatures. However, compared with the reduction of thermal conductivity, the improvement of power factor at low temperatures in n-type Te-doped Mg3Sb1.5Bi0.5 is much larger than those of p-type Mg3Sb2 and Mg3Sb1.8Bi0.2. Moreover, the thermal conductivity values of both p-type and n-type compounds at high temperatures are comparable, again confirming that the high thermoelectric performance of n-type Te-doped Mg3Sb1.5Bi0.5 mainly originates from the enhanced power factor.
Discussion
In summary, combining theory and experiment, we demonstrate in n-type Mg3Sb2-based Zintl compounds that multi-valley conduction band behaviour dominated by a light conduction band with 6 conducting carrier pockets leads to an enhanced Seebeck coefficient, a low resistivity and thereby a high power factor. Such a unique conduction band feature makes a key contribution to enhanced power factors and high zT over a wide temperature range, enabling Te-doped Mg3Sb1.5Bi0.5 to be a promising n-type thermoelectric candidate for low- and moderate-temperature applications. Our results thus provide an insightful guidance for the search for, and design of, high-performance n-type thermoelectric materials from Zintl compounds using multi-valley band engineering.
Methods
Sample synthesis
The samples with nominal compositions Mg3Sb1.5−0.5xBi0.5−0.5xTex (x=0.04, 0.05, 0.08 and 0.20) were synthesized by combining arc melting and spark plasma sintering (SPS) techniques. High-purity elements Sb pieces (99.9999%, Chempur), Bi pieces (99.999%, Chempur) and Te pieces (99.999%, Sigma Aldrich) were weighed, ground into powders (<100 μm) in an agate mortar and mixed in a ball mill mixer (SpectroMill, Chemplex Industries, Inc.) for 15 min. The mixed powders were then cold-pressed into pellets with the diameter of 12.7 mm. The pellets were completely melted by the arc melting process in an argon atmosphere using Edmund Bühler Mini Arc Melting System MAM-1GB. The arc melting process was repeated two times for both top and bottom sides of the pellets to obtain good homogeneity. The molten ingots were then ground into fine powders with particle sizes smaller than 63 μm and then mixed with Mg powders (99.8%, ≤44 μm, Alfa Aesar) in a ball mill mixer for 15 min. Approximately 2 g of the mixed powders were then loaded into a 12.7 mm diameter high-density graphite die protected by the graphite paper and sintered by SPS pressing under a pressure of 75 MPa. SPS sintering is conducted in vacuum by heating to 823 K in 11 min followed by a 2 min dwell, and then heating to 1123 K in 7 min and staying for another 4 min. SPS pressing was carried out using an SPS-515S instrument (SPS Syntex Inc., Japan).
Structure characterization
Powder X-ray diffraction measurements were carried out on the SPS-pressed pellets using a Rigaku Smartlab equipped with a Cu Kα source and parallel beam optic to check phase purity and lattice parameters (see Supplementary Note 1 and Supplementary Figs 3–5). Quantitative elemental analysis of the high-performance pellet with x=0.05 was carried out on FEI Nova Nano SEM 600 equipped with an element EDS X-ray detector. The result shown in Supplementary Table 1 was the average value from five randomly selected areas of the pellet.
Thermoelectric transport property measurements
The in-plane Hall coefficient (RH) and resistivity ρ were measured on the pellets using the Van der Pauw method in a magnetic field up to 1.25 T (ref. 29). Hall carrier concentration (nH) was calculated by 1/eRH, where e is the elementary charge. The Hall carrier mobility μH was then calculated using the relation  . The pellets were annealed by running the measurement during both heating and cooling for more than 3 cycles. After the first cycle, the Hall measurements of the pellets are consistent upon several repeated heating and cooling measurements. The resistivity and Hall data of the final cycle was used for the analysis. The Seebeck coefficients of the pellets were then measured from the slope of the thermopower versus temperature gradient using chromel-niobium thermocouples on an in-house system, similar to the one reported by Iwanaga et al.30 The thermal diffusivity (D) from 300 to 725 K was measured using the laser flash method (Netzsch, LFA457) (see Supplementary Fig. 7). Heat capacity (CP) was estimated using the Dulong–Petit law CP=3kB per atom for the temperature range from 300 to 725 K. The density (d) was measured by Archimedes method. Thermal conductivity was then calculated by κ=dDCP. For a comparison, one high-performance pellet Mg3Sb1.5−0.5xBi0.5−0.5xTex (x=0.05) was polished and cut into a 2 × 2 × 9 mm bar for the measurement of electrical transport properties, including electrical resistivity (ρ) and Seebeck coefficient (α), using a ZEM-3 (ULVAC) apparatus under a helium atmosphere from 300 to 725 K (Supplementary Fig. 8 and Supplementary Note 2). The thermoelectric zT, obtained on the high-performance sample with x=0.05 by the home-built system and ZEM-3 setup, shows good consistency between each other (Supplementary Fig. 9). The estimated measurement uncertainties are listed as follows: 5% for electrical resistivity, 5% for Seebeck coefficient and 7% for thermal diffusivity; the combined uncertainty for zT is ∼20%.
. The pellets were annealed by running the measurement during both heating and cooling for more than 3 cycles. After the first cycle, the Hall measurements of the pellets are consistent upon several repeated heating and cooling measurements. The resistivity and Hall data of the final cycle was used for the analysis. The Seebeck coefficients of the pellets were then measured from the slope of the thermopower versus temperature gradient using chromel-niobium thermocouples on an in-house system, similar to the one reported by Iwanaga et al.30 The thermal diffusivity (D) from 300 to 725 K was measured using the laser flash method (Netzsch, LFA457) (see Supplementary Fig. 7). Heat capacity (CP) was estimated using the Dulong–Petit law CP=3kB per atom for the temperature range from 300 to 725 K. The density (d) was measured by Archimedes method. Thermal conductivity was then calculated by κ=dDCP. For a comparison, one high-performance pellet Mg3Sb1.5−0.5xBi0.5−0.5xTex (x=0.05) was polished and cut into a 2 × 2 × 9 mm bar for the measurement of electrical transport properties, including electrical resistivity (ρ) and Seebeck coefficient (α), using a ZEM-3 (ULVAC) apparatus under a helium atmosphere from 300 to 725 K (Supplementary Fig. 8 and Supplementary Note 2). The thermoelectric zT, obtained on the high-performance sample with x=0.05 by the home-built system and ZEM-3 setup, shows good consistency between each other (Supplementary Fig. 9). The estimated measurement uncertainties are listed as follows: 5% for electrical resistivity, 5% for Seebeck coefficient and 7% for thermal diffusivity; the combined uncertainty for zT is ∼20%.
DFT calculations
DFT calculations were carried out using a full-potential linear augmented plane-wave plus local orbitals method as implemented in the Wien2k code31. The relaxed structure parameters of Mg3X2 (X=As, Sb, Bi) from our previous work4 were adopted. The electronic structure calculations were conducted using the TB-mBJ potential32 to get accurate band gaps and spin–orbit coupling was included in the calculations. The plane wave cutoff parameter RMTKmax was set to 9 and the corresponding Brillouin zone was sampled by a 36 × 36 × 24 k mesh. An energy convergence criterion of 10−4 eV was used. The Fermi surface was plotted with the program Xcrysden33. For the anisotropic ML conduction band, the effective masses were calculated to be mxx=0.55me, myy*=0.21me and mzz*=0.28me (see Supplementary Note 3 for details). The conduction band at the K point was nearly isotropic with mxx*=myy*=0.32me and mzz*=0.21me. For the highly anisotropic Γ valence band, mxx*=myy*=1.15me and mzz*=0.15me. Electrical transport property calculations of Mg3Sb2 (the DFT curves in Figs 2a,b and 3c) were carried out by combining the ab initio band structure calculations and the Boltzmann transport theory under the constant carrier scattering time approximation as implemented in the BoltzTraP code34 (Supplementary Note 3 and Supplementary Fig. 10). To calculate the power factor curves of Mg3Sb2 shown in Fig. 2b, we need to estimate the carrier scattering time. Calculation details of the constant carrier scattering time τ are provided in Supplementary Note 4. For the discussion on Fig. 3d, the band structure was assumed to be rigid and independent of the temperature.
Band structure calculations of Mg3Sb2−xBix (x=0.5, 1 and 1.5) solid solutions were carried out in supercells with 40 atoms (2 × 2 × 2 unit cell). Crystal structures including lattice constants and ionic positions were fully relaxed using the PBE functional35 in the Vienna ab initio simulation package (VASP)36. The plane-wave energy cutoff was set at 400 eV. The energy and Hellmann–Feynman force convergence criteria were 10−4 eV and 0.01 eV Å−1, respectively. A 6 × 6 × 4 Monkhorst–Pack k mesh was used for crystal structure optimization. In Mg3Sb2−xBix solid solutions, there are 16 equivalent positions for Sb/Bi atoms, and thus the possible atomic concentrations of Bi are multiples of 1/16. The crystal structure of the supercell with the lowest energy was used for further calculations and analysis. Electronic structures of solid solutions including spin–orbit coupling effect were calculated by TB-mBJ potential32 in Wien2k31 code. The zone samplings were done with uniform 15 × 15 × 8 k mesh grids. Effective band structures of Mg3Sb2−xBix were calculated by unfolding the band structures of supercells into the primitive cells using an effective band unfolding technique37,38. Calculation details of the band unfolding and defect formation energy are shown in the Supplementary Notes 5 and 6.
Data availability
The data that support these findings are available from the corresponding author on request.
Additional information
How to cite this article: Zhang, J. et al. Discovery of high-performance low-cost n-type Mg3Sb2-based thermoelectric materials with multi-valley conduction bands. Nat. Commun. 8: 13901 doi: 10.1038/ncomms13901 (2017).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Snyder, G. J. & Toberer, E. S. Complex thermoelectric materials. Nat. Mater. 7, 105–114 (2008).
Heremans, J. P. et al. Enhancement of thermoelectric efficiency in PbTe by distortion of the electronic density of states. Science 321, 554–557 (2008).
Pei, Y. et al. Convergence of electronic bands for high performance bulk thermoelectrics. Nature 473, 66–69 (2011).
Zhang, J. et al. Designing high-performance layered thermoelectric materials through orbital engineering. Nat. Commun. 7, 10892 (2016).
Liu, W. et al. Convergence of conduction bands as a means of enhancing thermoelectric performance of n-type Mg2Si1−xSnx solid solutions. Phys. Rev. Lett. 108, 166601 (2012).
Zhang, J. et al. High-performance pseudocubic thermoelectric materials from non-cubic chalcopyrite compounds. Adv. Mater. 26, 3848–3853 (2014).
Biswas, K. et al. High-performance bulk thermoelectrics with all-scale hierarchical architectures. Nature 489, 414–418 (2012).
Christensen, M. et al. Avoided crossing of rattler modes in thermoelectric materials. Nat. Mater. 7, 811–815 (2008).
Zhao, L.-D. et al. Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals. Nature 508, 373–377 (2014).
Liu, H. L. et al. Copper ion liquid-like thermoelectrics. Nat. Mater. 11, 422–425 (2012).
Sales, B. C., Mandrus, D. & Williams, R. K. Filled skutterudite antimonides: a new class of thermoelectric materials. Science 272, 1325–1328 (1996).
Saramat, A. et al. Large thermoelectric figure of merit at high temperature in Czochralski-grown clathrate Ba8Ga16Ge30 . J. Appl. Phys. 99, 023708 (2006).
Snyder, G. J., Christensen, M., Nishibori, E., Caillat, T. & Iversen, B. B. Disordered zinc in Zn4Sb3 with phonon-glass and electron-crystal thermoelectric properties. Nat. Mater. 3, 458–463 (2004).
Condron, C. L., Kauzlarich, S. M., Gascoin, F. & Snyder, G. J. Thermoelectric properties and microstructure of Mg3Sb2 . J. Solid State Chem. 179, 2252–2257 (2006).
Bhardwaj, A. & Misra, D. K. Enhancing thermoelectric properties of a p-type Mg3Sb2-based Zintl phase compound by Pb substitution in the anionic framework. RSC Adv. 4, 34552–34560 (2014).
Bhardwaj, A. et al. Mg3Sb2-based Zintl compound: a non-toxic, inexpensive and abundant thermoelectric material for power generation. RSC Adv. 3, 8504–8516 (2013).
Ponnambalam, V. & Morelli, D. On the thermoelectric properties of Zintl compounds Mg3Bi2−xPnx (Pn=P and Sb). J. Electron. Mater. 42, 1307–1312 (2013).
Shuai, J. et al. Thermoelectric properties of Na-doped Zintl compound: Mg3−xNaxSb2 . Acta Mater. 93, 187–193 (2015).
Kim, S. et al. Thermoelectric properties of Mn-doped Mg-Sb single crystals. J. Mater. Chem. A 2, 12311–12316 (2014).
Bjerg, L., Madsen, G. K. H. & Iversen, B. B. Ab initio calculation of intrinsic point defects in ZnSb. Chem. Mater. 24, 2111–2116 (2012).
Wang, X.-J. et al. Synthesis and high thermoelectric efficiency of Zintl phase YbCd2−xZnxSb2 . Appl. Phys. Lett. 94, 092106 (2009).
Zhang, H. et al. Thermoelectric properties of Eu(Zn1−xCdx)2Sb2 . Dalton Trans. 39, 1101–1104 (2010).
Liu, W.-S. et al. Thermoelectric property studies on Cu-doped n-type CuxBi2Te2.7Se0.3 nanocomposites. Adv. Energy Mater. 1, 577–587 (2011).
Hsu, K. F. et al. Cubic AgPbmSbTe2+m: bulk thermoelectric materials with high figure of merit. Science 303, 818–821 (2004).
Shi, X. et al. Multiple-filled skutterudites: high thermoelectric figure of merit through separately optimizing electrical and thermal transports. J. Am. Chem. Soc. 133, 7837–7846 (2011).
Pei, Y., Lensch-Falk, J., Toberer, E. S., Medlin, D. L. & Snyder, G. J. High thermoelectric performance in PbTe due to large nanoscale Ag2Te precipitates and La doping. Adv. Funct. Mater. 21, 241–249 (2011).
Goldsmid, H. J. Thermoelectric Refrigeration Plenum (1964).
Nolas, G. S., Sharp, J. & Goldsmid, H. J. Thermoelectrics: Basic Principles and New Materials Developments Springer (2001).
Borup, K. A. et al. Measurement of the electrical resistivity and Hall coefficient at high temperatures. Rev. Sci. Instrum. 83, 123902 (2012).
Iwanaga, S., Toberer, E. S., LaLonde, A. & Snyder, G. J. A high temperature apparatus for measurement of the Seebeck coefficient. Rev. Sci. Instrum. 82, 063905 (2011).
Blaha, P., Schwarz, K., Madsen, G. K. H., Kvasnicka, D. & Luitz, J. An Augmented Plane Wave and Local Orbitals Program for Calculating Crystal Properties Technical University of Wien (2001).
Tran, F. & Blaha, P. Accurate band gaps of semiconductors and insulators with a semilocal exchange-correlation potential. Phys. Rev. Lett. 102, 226401 (2009).
Kokalj, A. Computer graphics and graphical user interfaces as tools in simulations of matter at the atomic scale. Comput. Mater. Sci. 28, 155–168 (2003).
Madsen, G. K. H. & Singh, D. J. BoltzTraP. A code for calculating band-structure dependent quantities. Comput. Phys. Commun. 175, 67–71 (2006).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Popescu, V. & Zunger, A. Extracting E versus k effective band structure from supercell calculations on alloys and impurities. Phys. Rev. B 85, 085201 (2012).
Rubel, O., Bokhanchuk, A., Ahmed, S. J. & Assmann, E. Unfolding the band structure of disordered solids: From bound states to high-mobility Kane fermions. Phys. Rev. B 90, 115202 (2014).
Acknowledgements
This work was supported by the Danish National Research Foundation (DNRF93) and the Danish Center for Scientific Computing. We thank N. Pryds of Technical University of Denmark for the use of ZEM-3 setup. We thank K.A. Borup, K.F.F. Fischer, H. Reardon and A.B. Blichfeld for discussions.
Author information
Authors and Affiliations
Contributions
J.Z. and B.B.I. designed the study. J.Z., L.S. and S.H.P synthesized samples, characterized structures and measured high-temperature thermoelectric properties. J.Z. performed theoretical calculations. H.Y. helped in some thermal transport measurements and provided discussions. L.T.H. helped in the electrical transport measurements. J.Z., L.S. and B.B.I. analyzed data. J.Z. and B.B.I. wrote the manuscript. All other authors read and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures, Supplementary Table, Supplementary Notes and Supplementary References. (PDF 809 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhang, J., Song, L., Pedersen, S. et al. Discovery of high-performance low-cost n-type Mg3Sb2-based thermoelectric materials with multi-valley conduction bands. Nat Commun 8, 13901 (2017). https://doi.org/10.1038/ncomms13901
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms13901
This article is cited by
-
Efficient first-principles electronic transport approach to complex band structure materials: the case of n-type Mg3Sb2
npj Computational Materials (2024)
-
Leveraging language representation for materials exploration and discovery
npj Computational Materials (2024)
-
Full-landscape selection rules of electrons and phonons and temperature-induced effects in 2D silicon and germanium allotropes
npj Computational Materials (2024)
-
Roles of Cu doping in YbZn2Sb2 for thermoelectric performance enhancement
Rare Metals (2024)
-
Improved figure of merit (z) at low temperatures for superior thermoelectric cooling in Mg3(Bi,Sb)2
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.