Abstract
FOXP3+ Regulatory T (Treg) cells play a key role in the maintenance of immune homeostasis and tolerance. Disruption of Foxp3 expression results in the generation of instable Treg cells and acquisition of effector T-cell-like function. Here we report that the E3 deubiquitinase USP21 prevents the depletion of FOXP3 at the protein level and restricts the generation of T-helper-1-like Treg cells. Mice depleted of Usp21 specifically in Treg cells display immune disorders characterized by spontaneous T-cell activation and excessive T-helper type 1 (Th1) skewing of Treg cells into Th1-like Treg cells. USP21 stabilizes FOXP3 protein by mediating its deubiquitination and maintains the expression of Treg signature genes. Our results demonstrate how USP21 prevents FOXP3 protein depletion and controls Treg lineage stability in vivo.
Similar content being viewed by others
Introduction
FOXP3+ Regulatory T (Treg) cells have immune suppressive capacity and are crucial for the maintenance of immune homeostasis and control of dominant immune tolerance1,2,3. Therefore, it is of paramount importance to understand the mechanism underlying lineage stability of Treg cells in vivo. Debate over the stability of FOXP3+ Treg cells has arisen from disparate conclusions of previous studies4,5,6,7,8. In these studies, instable Treg cells acquire a T-effector-cell-like phenotype in response to inflammatory or lymphopenic cues, and also found to have instable FOXP3 expression5,9,10,11. Instable Treg cells produce higher amounts of inflammatory cytokines, which positively correlates with autoimmunity5,10,12,13.
The immunosuppressive phenotype of Treg cells is largely determined by gene expression patterns driven by FOXP3 (refs 2, 3, 14). Compromised epigenetic programing at the Foxp3 gene locus abrogates its gene transcription and facilitates the generation of exFOXP3 T cells5,15,16,17,18. These exFOXP3 T cells may produce inflammatory cytokines that lead to the rapid onset of autoimmune diseases5,10. In addition to the transcriptional control of the Foxp3 gene, the stability of FOXP3 expression is also determined at the post-translational level. For example, Treg cells respond to stress signals elicited by proinflammatory cytokines and lipopolysaccharides by degrading FOXP3 protein to then acquire a T-effector-cell-like phenotype19,20,21. Thus, the direct tracing of FOXP3 protein and its stability in vivo would contribute to the better understanding of instable Treg cells and their physiological role in health and disease.
Usp21 conventional knockout mice develop splenomegaly and spontaneous T-cell activation22,23, suggesting a potential role of USP21 in maintaining immune tolerance. We previously identified how the E3 deubiquitinase USP21 is highly induced in human CD4+CD25hiCD127lo Treg cells from asthma patients24, but the in vivo function of USP21 remained unclear.
To illustrate the function of USP21 in vivo, we generate mice with conditional depletion of Usp21 in Treg cells to investigate the role of USP21 in controlling Treg-cell stability. We find that mice lacking USP21 in Treg cells suffer from immune disorders characterized by spontaneous T-cell activation and excessive T-helper type 1 (Th1) skewing. Moreover, Treg-specific deletion of Usp21 leads to significant induction of Th1-like Treg cells. USP21 stabilizes FOXP3 protein by mediating its deubiquitination and maintains the expression of Treg signature genes. Taken together, our results show that USP21 prevents FOXP3 protein depletion and controls Treg lineage stability in vivo.
Results
Depletion of Usp21 in Treg cells perturbs immune tolerance
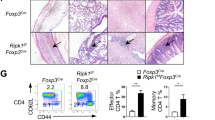
To illustrate the function of USP21 in controlling Treg-cell fate in vivo, we developed a mouse model where Usp21 is depleted only in Treg cells (Usp21fl/flFoxp3Cre mice) by crossing Usp21fl/fl mice with mice bearing YFP-fused Cre recombinase under control of the Foxp3 gene locus (Fig. 1a). We first analysed thymic development of CD4+ and CD8+ T cells, and no significant difference was observed between Foxp3Cre and Usp21fl/flFoxp3Cre mice (Supplementary Fig. 1a,b). Meanwhile, we did not find significant changes in the absolute numbers of CD4+CD8− (CD4-SP), CD4−CD8+ (CD8-SP), CD4−CD8− (DN) and CD4+CD8+ (DP) thymocytes in Usp21fl/flFoxp3Cre mice (Supplementary Fig. 1c). Usp21fl/flFoxp3Cre mice developed lymphadenopathy and splenomegaly at the age of 6 to 8 months (Fig. 1b,c), suggesting aberrant immune activation. We next tested whether Treg-specific deletion of Usp21 perturbed T-cell activation and homeostasis. We observed increased frequency of CD62LloCD44hi effector memory T cells in Usp21fl/flFoxp3Cre mice (Fig. 1d,e). We also observed excessive Th1 responses in Usp21fl/flFoxp3Cre mice, where splenic CD4+YFP− effector T cells (Teff) produced higher levels of interferon (IFN)-γ on in vitro stimulation (Fig. 1f,g). Therefore, USP21-deficient Treg cells failed to maintain immune tolerance and the related inflammation in vivo.
(a) CD4+CD25−YFP− effector T (Teff) cells and CD4+CD25hiYFP+ Treg cells were sorted from Foxp3Cre (WT) and Usp21fl/flFoxp3Cre (KO) mice. mRNA expression of Usp21 in each population was assessed by qRT–PCR. (n=3 for each group). (b) Image of spleens and peripheral lymph nodes (pLNs) from 8-month-old WT and KO mice. (c) Quantitative analysis of the weight of spleens isolated from WT (n=5) and KO (n=5) mice. (d) Representative figure shown the expression of CD62L and CD44 in splenic CD4+YFP− and CD8+ T cells from WT (n=7) and KO (n=7) littermates. (e) Percentage of splenic CD62LloCD44hi effector memory T cells as in d. (f) Representative figure shown the expression of IFN-γ, IL-17, IL-2 and IL-4 by splenic CD4+YFP− effector T (Teff) cells from WT (n=5) and KO (n=5) mice. (g) Percentage of IFN-γ+, IL-17+, IL-2+ and IL-4+ splenic T cells as in f. Small horizontal lines indicate the mean (±s.d.). All data represent means±s.d. **P≤0.01, as determined by Student’s t-test.
USP21-ΔTreg cells acquire a Th1-like phenotype
Further histological analysis revealed increased lymphocytic infiltration into peripheral organs of Usp21fl/flFoxp3Cre mice, including the liver, lung and salivary glands (Fig. 2a). We next analysed the expression of inflammatory cytokines produced by T effector and Treg cells at inflamed tissue loci. We observed the increased production of IFN-γ by CD4+YFP− effector T cells in the liver, lung and salivary glands of Usp21fl/flFoxp3Cre mice (Fig. 2b,d), revealing aberrant immune activation and severe selective Th1-type inflammation. Although the expression of IL-17A slightly increased in the lung of Usp21fl/flFoxp3Cre mice, its expression was much lower than IFN-γ (Fig. 2b,d). We found that inflamed tissue-derived CD4+YFP+ USP21-ΔTreg cells produced high amounts of IFN-γ and displayed a Th1-cell-like phenotype (Fig. 2c,e).
(a) Histological analysis of lymphocytic infiltration in the liver, lung and salivary glands of indicated mice by H&E staining. Scale bar, 100 μm. (b) Representative figure shown the expression of IFN-γ and IL-17A among CD4+YFP− effector T (Teff) cells in the liver, lung and salivary glands of WT (n=5) and KO (n=5) mice. (c) Representative figure shown the expression of IFN-γ and IL-17A among CD4+YFP+ Treg cells in the liver, lung and salivary glands of WT (n=5) and KO (n=5) mice. (d) Percentage of IFN-γ+ and IL-17A+ Teff cells as shown in b. (e) Percentage of IFN-γ+ and IL-17A+ Treg cells as shown in c. All data represent means±s.d. *P≤0.05, **P≤0.01, as determined by Student’s t-test. NS, not significant.
Naive CD4+ T cells were further sorted from wild type (WT) and Usp21fl/flFoxp3Cre mice and differentiated into Th1, Th17 and iTreg cells under polarizing conditions. We observed an increased production of IFN-γ in iTreg cells from Usp21fl/flFoxp3Cre mice (Supplementary Fig. 2a), confirming that USP21-ΔTreg cells displayed a Th1-like phenotype. However, the production of both IFN-γ and IL-17A was comparable in polarized Th1 and Th17 cells from WT and Usp21fl/flFoxp3Cre mice (Supplementary Fig. 2a). Therefore, Th1 skewing in Usp21fl/flFoxp3Cre mice is potentially due to aberrant function of Th1-like USP21-ΔTreg cells. In summary, we observed a population of Th1-like USP21-ΔTreg cells, which correlated with Th1-type inflammation in the liver, lung and salivary glands of Usp21fl/flFoxp3Cre mice.
USP21 prevents the loss of FOXP3 protein in Treg cells
To investigate the stability of USP21-ΔTreg cells, we next analysed the expression of FOXP3 using flow cytometry. We observed the downregulation of FOXP3 protein in USP21-ΔTreg cells from lymphoid as well as non-lymphoid organs (Fig. 3a), while the Foxp3 gene was still actively transcribed (Supplementary Fig. 3a). This suggested that the loss of USP21 affected the post-translational modification-mediated degradation of FOXP3 protein in these USP21-ΔTreg cells. Further testing indicated that USP21 is required to stabilize FOXP3 protein, since the mean fluorescence intensity of FOXP3 staining was downregulated in USP21-ΔTreg cells (Fig. 3b). More importantly, the percentages of CD4+YFP+ USP21-ΔTreg cells remained unaffected (Supplementary Fig. 3b–d), reflecting a normal distribution of Treg cells in the lymphoid as well as non-lymphoid organs of Usp21fl/flFoxp3Cre mice. Decrease in the percentages of FOXP3+ Treg cells was more pronounced in non-lymphoid organs, due to increased loss of FOXP3 protein expression (Fig. 3c). We next checked the expression of FOXP3 in CD4+YFP+ Treg cells and confirmed the instability of FOXP3 protein in CD4+YFP+ USP21-ΔTreg cells (Fig. 4a,b). To further test the in vivo stability of USP21-ΔTreg cells, we transferred WT Treg or USP21-ΔTreg cells into Rag1−/− mice. There was a significant loss of FOXP3 in in vivo transferred USP21-ΔTreg cells (Fig. 4c–e). Taken together, these results indicated that USP21 might control Treg lineage stability by preventing the loss of FOXP3 protein.
(a) Representative figure shown the expression of FOXP3 protein in CD4+ T cells from the thymus, spleen, pLNs, liver, lung and salivary glands of WT (n=6) and KO (n=5) mice. FOXP3 expression in CD4+ T cells was shown as histograms and FOXP3+ cells were further gated. The percentage of CD4+FOXP3+ T cells and mean fluorescent intensity (MFI) of FOXP3 from WT and KO mice were shown in blue (WT) and red (KO). (b) MFI of FOXP3 among FOXP3+ Treg cells indicated in a. (c) Percentage of FOXP3+ Treg cells as indicated in a. All data represent means±s.d. *P≤0.05, **P≤0.01, ***P≤0.001, as determined by Student’s t-test. NS, not significant.
(a) Representative figure shown the expression of FOXP3 protein in CD4+YFP+ Treg cells from the thymus, spleen, pLNs, liver, lung and salivary glands of WT (n=5), and KO (n=5) mice. FOXP3 expression in CD4+YFP+ T cells was shown as histograms. (b) Mean fluorescent intensity (MFI) of FOXP3 among CD4+YFP+ Treg cells indicated in a. (c) WT Treg or USP21-ΔTreg cells were transferred into Rag1−/− mice. The expression of FOXP3 protein was further analysed in transferred Treg cells from the spleen of Rag1−/− mice 7 days later. (d) Percentage of FOXP3+ Treg cells as indicated in c. WT group (n=6) and KO group (n=5), data represent means±s.d. (e) Mean fluorescent intensity (MFI) of FOXP3 among transferred Treg cells as indicated in c. WT group (n=6) and KO group (n=5), data represent means±s.d. **P≤0.01, ***P≤0.001, as determined by Student’s t-test. NS, not significant.
Depletion of Usp21 perturbs Treg signature gene expression
We performed RNA sequencing and compared gene expression profiles of Treg cells from Usp21fl/flFoxp3Cre and Foxp3Cre mice. We first compared differentially expressed genes (DEGs) of Treg and Teff signature genes25. We found that USP21-ΔTreg cells displayed impaired transcription of Treg signature genes and simultaneously acquired the expression of genes controlling Teff cell fate (Fig. 5a,b), supporting that USP21-ΔTreg cells became Teff-like. Moreover, in WT and USP21-ΔTreg cells, 35.4% (87 upregulated genes and 64 downregulated genes) of 426 DEGs were FOXP3 direct targets (Fig. 5c), where total FOXP3 targeting genes were taken from published CHIP-seq data14. The expression of representative genes was further validated by qRT–PCR (Supplementary Fig. 4a). In summary, these data suggest that USP21 regulates the function of Treg cells majorly through FOXP3.
(a) Scatter plot of genes significantly upregulated (yellow), downregulated (blue) or unchanged in USP21-ΔTreg cells. The cutoff value was set at fold change≥2 and P value<2.2e−16. (b) Clustering of differentially expressed genes identified in USP21-ΔTreg cells compared with WT Treg cells. Yellow and purple represent high and low levels of expression of the indicated genes, respectively. The colours indicate the value of log2 fold change. (c) Percentage of FOXP3-bound genes that changed in expression in USP21-ΔTreg cells, where published FOXP3 ChIP-seq data sets were used as total FOXP3 target gene coverage. (d) CD4+CD25−YFP− Teff cells were sorted from WT mice and labelled with CellTrace Violet. Labelled responder T cells were then either cultured alone or mixed with varying amounts (as indicated) of CD4+CD25hiYFP+ Treg cells sorted from WT or KO mice. Cell mixtures were stimulated with anti-CD3/CD28 beads and the proliferation of the responder cells was measured three days later. (e) Percentage of proliferated responder T cells was assessed as shown in d. (n=3 for each group). All data represent means±s.d. *P≤0.05, **P≤0.01, as determined by Student’s t-test. NS, not significant.
USP21-ΔTreg cells display impaired suppressive activity
We next examined the suppressive activity of USP21-ΔTreg cells using an in vitro suppression assay. We found that USP21-ΔTreg cells had significantly impaired suppressive capacity towards Teff cell proliferation (Fig. 5d,e). Knockdown of Usp21 in WT Treg cells also impaired their suppressive activity (Supplementary Fig. 4b–d), confirming that USP21 is required for Treg-cell function. These results collectively suggest that USP21 maintains the expression of Treg signature genes and controls the suppressive function of Treg cells.
We further challenged Usp21fl/flFoxp3Cre mice with MOG peptide in an experimental allergic encephalomyelitis (EAE) disease model. We found that Usp21fl/flFoxp3Cre mice developed more severe EAE symptoms and failed to restrict late stages of disease development (Fig. 6a; Table 1). Disease severity was accompanied by increased production of IFN-γ and IL-17A in CD4+YFP− effector T cells from Usp21fl/flFoxp3Cre mice (Fig. 6b,c). We then transferred CD45.2+ WT Treg or USP21-ΔTreg cells into EAE-bearing CD45.1 mice, and CD45.2+ USP21-ΔTreg cells became instable through FOXP3 loss (Fig. 6d,e). Therefore, USP21-ΔTreg cells fail to limit EAE disease development at later phases of disease progression, possibly due to the loss of FOXP3 expression and suppressive function.
(a) Clinical severity of EAE in WT (n=8) and KO (n=7) mice was monitored for 27 days after immunization with MOG peptide. Statistical analysis of the clinical signs was further described in Table 1. (b) Representative figure shown the expression of IFN-γ and IL-17A by CD4+YFP− effector T cells in the spleen, peripheral lymph nodes and CNS from WT (n=5) and KO (n=5) mice on day 27 post EAE induction. (c) Percentage of IFN-γ+ and IL-17A+ cells among CD4+YFP− effector T cells in the spleen, peripheral lymph nodes and CNS from WT (n=5) and KO (n=5) mice as indicated in b. (d) CD45.2+ WT Treg or USP21-ΔTreg cells were transferred intravenously into CD45.1 mice at the onset of EAE (day 12). At the peak of EAE (day 17), the transferred CD45.2+ Treg cells were analysed for FOXP3 expression in the CNS from the CD45.1 mice. (e) Mean fluorescent intensity (MFI) of FOXP3 among transferred CD45.2+ Treg cells as indicated in e. (n=5 for each group). All data represent means±s.d. *P≤0.05, **P≤0.01, ***P≤0.001, as determined by Student’s t-test. NS, not significant.
USP21 interacts with FOXP3 in Treg cells
Our data suggested that a post-translational modification-mediated mechanism controls the degradation of FOXP3 in USP21-ΔTreg cells (Fig. 3a; Supplementary Fig. 3a). To test whether USP21 is a direct E3 deubiquitinase of FOXP3 that prevents its degradation, we first carried out binding studies to determine whether FOXP3 could directly bind to USP21. Reciprocal immunoprecipitation of FLAG-FOXP3 and Myc-USP21 revealed an interaction between USP21 and FOXP3 (Fig. 7a), and we also detected the endogenous interaction between USP21 and FOXP3 in human Treg cells (Fig. 7b). We next expressed and purified His-USP21, MBP and MBP-FOXP3 from Escherichia coli, and carried out an in vitro MBP pulldown assay that confirmed the direct interaction between FOXP3 and USP21 (Fig. 7c). Through the generation of systematic deletion mutants (Supplementary Fig. 5a) and co-IP experiments, we found that the zinc-finger subdomain of FOXP3 was essential for its interaction with USP21; deletion of the zinc-finger regions (N2, C1 and C2) disrupted their interaction (Supplementary Fig. 5b). These data further suggested a direct role of USP21 in Treg cells through interaction with FOXP3.
(a) Flag-FOXP3 and Myc-USP21 expression plasmids were cotransfected into 293T cells. Cells were lysed for co-IP as indicated. (b) Human CD4+CD25hiCD127lo expanding Treg cells were cultured with α-CD3 and α-CD28 antibodies in the presence of IL-2. Cells were collected for endogenous co-IP and western blotting. Cell lysates of Teff, Th17, iTreg and expanding Treg cells were loaded as input. (c) Purified His-USP21 and either MBP or MBP-FOXP3 were incubated together in a cell-free system. MBP pulldown assay was further performed followed by western blotting. (d) Polarized WT or KO iTreg cells were treated with or without MG132 for 4 h. Cell lysates were further analysed by western blotting. (e) HA-FOXP3 was expressed in HEK 293T cells with or without Myc-USP21. Cells were treated with 20 μg ml−1 cycloheximide (CHX) for the indicated periods. Cell lysates were further analysed by Western blotting. (f) Pulldown assay of his-ubiquitin conjugated Flag-FOXP3 as described in methods. (g) Naive CD4+ T cells were sorted from WT or KO mice and further polarized into iTreg cells. Cell lysates were immunoprecipitated with anti-Ubiquitin antibody and ubiquitinated FOXP3 levels were detected by western blotting.
USP21 deubiquitinates FOXP3 in Treg cells
We further found the loss of FOXP3 protein in USP21-ΔTreg cells could be prevented by the addition of proteasome inhibitor MG132 (Fig. 7d), suggesting that the ubiquitin-proteasome pathway might be involved in this process. We next studied the half-life of FOXP3 using the protein synthesis inhibitor cycloheximide. And ectopically expressed USP21 significantly extended the half-life of FOXP3 protein (Fig. 7e). Therefore, USP21 prevents the proteasomal degradation of FOXP3 in Treg cells.
Using a his-tagged ubiquitin pulldown assay, we found that ectopically expressed USP21, but not the enzymatically inactive mutant C221A, reduced levels of ubiquitinated FOXP3 (Fig. 7f). We also observed a much higher level of endogenous ubiquitinated FOXP3 in USP21-ΔTreg cells (Fig. 7g), correlating with the loss of FOXP3 protein in vivo. Therefore, these results support that USP21 prevents FOXP3 depletion in Treg cells through deubiquitination and protection from ubiquitination-mediated protein degradation.
To identify the specific lysine residues of FOXP3 that could be deubiquitinated by USP21, we screened each individual lysine-only mutants of FOXP3 (where all lysines in FOXP3 were mutated to arginines besides one residue) and identified seven lysine residues (K206, K216, K227, K252, K277, K332 and K393), which were potential USP21 targets. We then tested mutants where only seven lysine residues were retained or mutated into arginines (termed 7K and 7R mutants, respectively, Supplementary Fig. 5c). His-ubiquitin pulldown assays further confirmed that USP21 could deubiquitinate FOXP3 at these seven lysine residues and that the 7R construct was unresponsive to USP21-mediated deubiquitination (Supplementary Fig. 5d). These findings demonstrated that USP21 could stabilize FOXP3 through deubiquitination and prevented the depletion of FOXP3 protein in Treg cells.
USP21 is dispensable for GATA3 expression in murine Tregs
USP21 was previously reported to positively regulate GATA3 expression through deubiquitination in human Treg cells24. In agreement with this previous study, we confirmed the endogenous interaction between USP21 and GATA3 in human expanding Treg cells (Supplementary Fig. 6a). However, GATA3 protein remained stable in murine USP21-ΔTreg cells from the thymus, spleen and peripheral lymph nodes (Supplementary Fig. 6b). We also compared the expression level of GATA3 protein in sorted CD4+CD25hiYFP+ Treg cells or polarized iTreg cells from WT and Usp21fl/flFoxp3Cre mice, and no significant difference was observed (Supplementary Fig. 6c). These results are consistent with a previous study that showed how USP21 is dispensable for the regulation of GATA3 during murine lymphocyte differentiation23, perhaps suggesting the existence of a redundant role of USP21 in stabilizing GATA3 in mice, but not in humans.
Discussion
Ample evidence has supported the notion of how FOXP3 instability facilitates the generation of T-effector-cell-like Treg cells5,10,26,27,28,29. Through generation of Usp21fl/flFoxp3Cre mice, we observed a population of Th1-like instable USP21-ΔTreg cells, which was accompanied by increased Th1 responses. This was due to increase in degradation of FOXP3 through the lack of deubiquitinase activity. These observations are consistent, albeit from an opposite mechanistic standpoint, with a previous finding that Stub1-overexpressing Treg cells became Th1-cell-like phenotype due to increased FOXP3 depletion via ubiquitination and proteasome-mediated degradation19. Moreover, in human patients with relapsing remitting multiple sclerosis (RRMS), a high frequency of IFN-γ-secreting Treg cells has been observed26, confirming the existence of Th1-like Treg cells in humans. There have also been reports of how Treg cells with decreased FOXP3 expression preferentially become Th2-type effectors in hosts with severe Th2-type disorder12. In addition, under arthritic conditions, CD25loFOXP3+CD4+ T cells may lose FOXP3 expression and undergo transdifferentiation into Th17 cells13. Therefore, instable Treg cells may adopt various T-helper-cell-like phenotypes under different inflammatory contexts.
K48-linked polyubiquitinated FOXP3 is degraded in the proteasome through the action of the E3 ubiquitin ligase Stub1 (ref. 19). And another report also described how Stub1 and Cbl-b sequentially induce FOXP3 for ubiquitination and degradation30. Here USP21 prevents FOXP3 degradation most likely through removing K48-linked polyubiquitin moieties. Previously, another deubiquitinase, USP7, was also reported to stabilize FOXP3 through deubiquitination and improved Treg-cell functionality20. Therefore, these results collectively suggest that both deubiquitinases were required to maintain FOXP3 expression in Treg cells. In addition to FOXP3, USP21 might have additional targets in Treg cells, since a proportion of DEGs were not controlled directly by FOXP3. However, our data suggest that FOXP3 should be an important target of USP21 in Treg cells, since FOXP3 critically controls Treg-cell development and functional stability.
USP21-ΔTreg cells had compromised expression of Treg signature genes and displayed impaired suppressive activity. In an EAE disease model, we found that USP21-ΔTreg cells failed to restrict both Th1 and Th17 responses, possibly due to the loss of FOXP3 expression and subsequent loss of suppressive activity. Therefore, USP21-ΔTreg cells are largely hypoactive and failed to restrict the activation of inflammatory lymphocytes in lymphoid and non-lymphoid organs. Although CD4+YFP− effector T cells produced IFN-γ at much higher levels in Usp21fl/flFoxp3Cre mice, Th1-like USP21-ΔTreg cells could potentially contribute to disease pathology by amplifying Th1 responses through IFN-γ production and impaired effector-T-cell-mediated Th1 responses. To really understand whether this is the case, dual depletion of USP21 and effector-T-cell cytokines such as IFN-γ in Treg cells might be required.
Treg cells are known to restrict anti-tumour immune responses and promote tumour survival31,32,33,34,35. Therefore, temporary release from the suppressive influence of Treg cells or conversion of these cells into Th1-like inflammatory cells might provide beneficial traits in the tumour microenvironment to support anti-tumour responses. Interestingly, a recent study has characterized a population of FOXP3lo T cells in colorectal cancer, which produced higher amounts of IFN-γ after in vitro stimulation, also predicted better prognosis36. Therefore, Th1-like FOXP3lo Treg cells potentially contribute to anti-tumour immune responses and suppress tumour formation. Here the induction of FOXP3 degradation in Treg cells provides a potential method that enables transient inhibition of suppressive Treg cells, and thus USP21 is a potential drug target for future anti-cancer immunotherapy (Fig. 8).
In Treg cells, FOXP3 activates the transcription of Usp21 gene upon TCR stimulation. USP21 prevents the degradation of FOXP3 through deubiquitination and therefore controls the stability of Treg cells. Moreover, USP21-ΔTreg cells confer a Th1-like phenotype and facilitate Th1 responses in the host. Therefore, depletion of FOXP3hi Treg cells by using USP21 as the drug target potentially offers promise for anti-cancer immunotherapies.
Methods
Mice
The mouse line carrying the Usp21tm1a(EUCOMM)Wtsi allele was purchased from the International Mouse Knockout Consortium. To generate Usp21flox/flox mice, Usp21tm1a(EUCOMM)Wtsi mice were further crossed with Flp recombinase transgenic mice of C57BL/6 strain. Foxp3YFP-Cre mice were purchased from the Jackson Laboratory. Female Foxp3Cre (WT) and Usp21fl/flFoxp3Cre (KO) mice of C57BL/6 strain were used at 6- to 8-month old, unless otherwise noted. Randomization and blinding strategy was used whenever possible. All mice were maintained under specific pathogen-free conditions. All mouse experiments followed protocols approved by the Institutional Animal Care and Use Committee in Institut Pasteur of Shanghai.
Antibodies
Labelled anti-CD4 (GK1.5, 1:100), anti-CD8 (53-6.7, 1:100), anti-CD25 (PC61, 1:100), anti-CD44 (IM7.8.1, 1:100), anti-CD62L (MEL14, 1:100), anti-FOXP3 (FJK-16s, 1:100), anti-GATA3 (L50-823, 1:100), anti-IL-2 (JES6-5H4, 1:100), anti-IL-4 (BVD4-1D11, 1:100), anti-IL-17A (TC11-18H10, 1:100), anti-IFN-γ (XMG1.2, 1:100) and specific isotype-matched control antibodies (1:100) were from BD Biosciences, eBioscience or Miltenyl Biotec. Anti-USP21 (G-17, 1:1,000), Anti-Ubiquitin (P4D1, 1:1,000), Anti-GATA3 (HG3-31, 1:1,000) was from Santa Cruz Biotechnology. Anti-FOXP3 (eBio7979, 1:1,000) was from eBioscience.
Flow cytometry
For analysis of surface markers, cells were stained in PBS containing 2% fetal bovine serum (FBS) with antibodies as indicated. FOXP3 staining was performed according to the manufacturer’s instructions (eBioscience). To determine cytokine expression, cells were stimulated with phorbol12-myristate 13-acetate (50 ng ml−1), ionomycin (1 μM), Golgi Stop and Golgi Plug for 4 h. At the end of stimulation, cells were stained with fixable viability dye eFluor 780 and the indicated antibodies according to the manufacturer’s instructions (eBioscience).
Cell purification and culture
HEK 293T (ATCC CRL-11268) cells were cultured in DMEM containing 10% FBS and tested for mycoplasma contamination before use. Lymphocytes were isolated from lymphoid organs (spleen and peripheral lymph nodes) and CD4+ T cells were enriched with isolation kits. CD4+CD25hiYFP+ Treg cells and CD4+CD25−YFP− Teff cells were further sorted on a BD FACS ARIA II sorter (BD Biosciences). Sorted Treg and Teff cells were cultured in RPMI 1640 medium (plus β-mercaptoethanol) supplemented with 10% FBS (GIBCO), 1% GlutaMax (GIBCO), 1% sodium pyruvate (GIBCO), and 1% Pen/Strep (GIBCO). For Usp21 knockdown assay, murine CD4+CD25hiYFP+ Treg cells were transduced with virus containing shRNA lentiviral vector, along with anti-CD3/28 stimuli (1 cell to 1 bead). The following shRNA sequences were used: shCK, 5′-caacaagatgaagagcaccaa-3′; shUsp21-1, 5′-ccagaaatacgtcccttcctt-3′; shUsp21-2, 5′-cccagatgaaaggctcaagaa-3′. Mice CD4+CD25loYFP−CD62L+ naïve T cells were sorted and further polarized into iTreg cells using anti-CD3/CD28 DynaBeads (Invitrogen) in the presence of mIL-2 (100 U ml−1) plus mTGF-β1 (5 ng ml−1, R&D). For Th1 conditions, 10 ng ml−1 IL-12 and 10 μg ml−1 anti-IL-4 were added. For Th17 conditions, 10 μg ml−1 anti-IL-4, 10 μg ml−1 anti-IFN-γ, 2 ng ml−1 mTGF-β1, 20 ng ml−1 IL-23 and 20 ng ml−1 IL-6 were added. To enrich lymphocytes from the liver, lung and salivary glands, mice were transcardially perfused with heparin (10 U ml−1) in PBS under anaesthesia. Tissues were excised independently of lymph nodes and were incubated for 1 h at 37 °C in Collagenase D (1 mg ml−1; Roche), DNase (20 mg ml−1, Roche) and 0.2% bovine serum albumin in RPMI 1640 medium. Digested tissue was strained through cell strainers. Lymphocytes were further separated by centrifugation through a 40–70% percoll gradient and then subjected to Flow Cytometry Analysis. Human CD4+CD25hiCD127lo Treg cells were isolated from PBMC by FACS on a BD FACS ARIA II sorter. And informed consent was obtained from healthy donors. All the studies were approved by the Institutional Ethics Committee. Human Treg cells were expanded using anti-CD3/CD28 DynaBeads (Invitrogen) at a cell to bead ratio of 1:3 in X-VIVO media (Lonza) supplemented with 10% FBS, 1% GlutaMax, 1% sodium pyruvate, and 1% Pen/Strep. Treg cells were expanded in the presence of rIL-2 (500 U ml−1) and rapamycin (100 nM) for 10 days followed by resting in a lower concentration of rIL-2 (100 U ml−1). Human CD4+CD25loCD45RAhi naive T cells were isolated from healthy donor PBMC and differentiated into iTreg cells using anti-CD3/CD28 DynaBeads (Invitrogen) in the presence of rhIL-2 (100 U ml−1) and hTGF-β1 (5 ng ml−1, R&D).
EAE model
Four-month old mice were immunized subcutaneously with 200 μl of emulsified CFA and 200 mg MOG35–55 peptide (MEVGWYRSPFSRVVHLYRNGK), and received intraperitoneal injections of 200 ng pertussis toxin at the time of immunization, and repeated 48 h later. Animals were evaluated daily for signs of disease by the following criteria: 0, no disease; 1, flaccid tail paralysis; 2, hind limb paresis; 3, bilateral hind limb paralysis; 4, fore and hind limb paralysis.
Adoptive transfer of Treg cells
CD4+CD25+YFP+ Treg cells were sorted from WT and KO mice. A total of 106 Treg cells were transferred intravenously into Rag1−/− mice. Rag1−/− mice were sacrificed 7 days later. A total of 2 × 106 CD45.2+ WT Treg cells or USP21-ΔTreg cells were transferred intravenously into CD45.1 mice at the onset of EAE (day 12). At the peak of EAE (day 17), the transferred CD45.2+ Treg cells were analysed for FOXP3 expression in the CNS from the CD45.1 mice.
RNA preparation and immunoblotting
Total RNA was extracted using TRIzol reagent (Invitrogen). cDNA was synthesized using a reverse transcriptase kit (TaKaRa, Japan), followed by qRT–PCR analysis (SYBR Green; TaKaRa). Cells were washed with ice-cold PBS and lysed on ice for 30 min in RIPA buffer (50 mM Tris-HCl, pH 7.5; 135 mM NaCl; 1% NP-40; 0.5% sodium DOC; 1 mM EDTA; 10% glycerol) containing protease inhibitor (1:100, P8340; Sigma-Aldrich), 1 mM NaF, and 1 mM PMSF. Cell lysates were cleared by centrifugation, and supernatants were immunoprecipitated with the appropriate antibodies (Abs, 1 μg ml−1) using protein A/G-agarose beads at 4 °C. Samples were then used for immunoblotting analysis with indicated antibodies.
His-ubiquitin pulldown assay
Cells were lysed in a pH 8 urea buffer (8 M urea, 100 mM Na2HPO4, 10 mM TRIS (pH 8.0), 0.2% TX-100, 10 mM imidazole and 1 mM N-ethylmaleimide) and incubated with Ni-NTA beads for 2 h at room temperature. The beads were washed twice in pH 8 urea buffer, twice in pH 6.3 urea buffer (8 M urea, 100 mM Na2HPO4, 10 mM TRIS (pH 6.3), 0.2% TX-100 and 10 mM imidazole), and once in a wash buffer (20 mM TRIS (pH 8.0), 100 mM NaCl, 20% glycerol, 1 mM dithiothreitol and 10 mM imidazole). Samples were then used for immunoblotting analysis with indicated antibodies.
MBP pulldown assay
MBP, MBP-FOXP3 and His-USP21 were expressed in Escherichia coli and purified by amylose resin or Ni-NTA beads. His-USP21 were incubated with MBP or MBP-FOXP3 protein for 2 h at 4 °C in buffer A (20 mM Tris·HCl (pH 7.5), 200 mM NaCl, 5 mM β-mercaptoethanol, and 1 mM PMSF) and then were incubated with amylose resins for another 2 h. Amylose resins then were washed extensively with buffer A, followed by SDS/PAGE.
RNA-Seq library generation
Total RNA was isolated using RNeasy mini kit (Qiagen, Germany). Strand-specific libraries were prepared using the TruSeq Stranded Total RNA Sample Preparation kit (Illumina, USA) following the manufacturer’s instructions. Briefly, mRNA was purified, fragmented and used for first- and second-strand cDNA synthesis followed by adenylation of 3′ ends. Samples were ligated to unique adaptors and subjected to PCR amplification. Libraries were quantified by Qubit 2.0 Fluorometer (Life Technologies, USA) and validated by Agilent 2100 bioanalyzer (Agilent Technologies, USA) to confirm the insert size and calculate the mole concentration. Cluster was generated by cBot with the library diluted to 10 pM and then were sequenced on the Illumina HiSeq 2500 (Illumina, USA). The library construction and sequencing was performed at Shanghai Biotechnology Corporation.
High-throughput sequencing and analysis
Sequencing raw reads were preprocessed by filtering out rRNA reads, sequencing adaptors, short-fragment reads and other low-quality reads. Tophat v2.0.9 was used to map the cleaned reads to the mouse mm10 reference genome with two mismatches. After genome mapping, Cufflinks v2.1.1 was run with a reference annotation to generate FPKM values for known gene models. Differentially expressed genes were identified using Cuffdiff. The P value significance threshold in multiple tests was set by the false discovery rate (FDR). The fold changes were also estimated according to the FPKM in each sample. The differentially expressed genes were selected using the following filter criteria: FDR ≤0.05 and fold change ≥2.
Statistical analysis
P values were calculated with Student’s t-test or analysis of variance (GraphPad Prism) as specified in figure legends. All data represent means±s.d. *P≤0.05, **P≤0.01, ***P≤0.001, as determined by two-tailed, unpaired Student’s t-test. NS, not significant. Sample sizes were designed with adequate power according to the literature and our previous studies. Randomization and blinding strategy was used whenever possible.
Data availability
The RNA-seq data that support the findings of this study are available in GEO database with the GEO accession number GSE77458. The uncropped scans of all western blots were supplied in Supplementary Figs 7 and 8. The authors declare that all the data supporting the findings of this study are available within the article and its Supplementary Information Files or from the corresponding author on reasonable request.
Additional information
How to cite this article: Li, Y. et al. USP21 prevents the generation of T-helper-1-like Treg cells. Nat. Commun. 7, 13559 doi: 10.1038/ncomms13559 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Khattri, R., Cox, T., Yasayko, S. A. & Ramsdell, F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4, 337–342 (2003).
Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4, 330–336 (2003).
Hori, S., Nomura, T. & Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 (2003).
Rubtsov, Y. P. et al. Stability of the regulatory T cell lineage in vivo. Science 329, 1667–1671 (2010).
Zhou, X. et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 10, 1000–1007 (2009).
Sakaguchi, S., Vignali, D. A., Rudensky, A. Y., Niec, R. E. & Waldmann, H. The plasticity and stability of regulatory T cells. Nat. Rev. Immunol. 13, 461–467 (2013).
Sawant, D. V. & Vignali, D. A. Once a Treg, always a Treg? Immunol. Rev. 259, 173–191 (2014).
Miyao, T. et al. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity 36, 262–275 (2012).
Tsuji, M. et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science 323, 1488–1492 (2009).
Bailey-Bucktrout, S. L. et al. Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity 39, 949–962 (2013).
Duarte, J. H., Zelenay, S., Bergman, M. L., Martins, A. C. & Demengeot, J. Natural Treg cells spontaneously differentiate into pathogenic helper cells in lymphopenic conditions. Eur. J. Immunol. 39, 948–955 (2009).
Wan, Y. Y. & Flavell, R. A. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature 445, 766–770 (2007).
Komatsu, N. et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med. 20, 62–68 (2014).
Samstein, R. M. et al. Foxp3 exploits a pre-existent enhancer landscape for regulatory T cell lineage specification. Cell 151, 153–166 (2012).
Zheng, Y. et al. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 463, 808–812 (2010).
Li, X., Liang, Y., LeBlanc, M., Benner, C. & Zheng, Y. Function of a Foxp3 cis-element in protecting regulatory T cell identity. Cell 158, 734–748 (2014).
Feng, Y. et al. Control of the inheritance of regulatory T cell identity by a cis element in the Foxp3 locus. Cell 158, 749–763 (2014).
Josefowicz, S. Z. et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature 482, 395–399 (2012).
Chen, Z. et al. The ubiquitin ligase Stub1 negatively modulates regulatory T cell suppressive activity by promoting degradation of the transcription factor Foxp3. Immunity 39, 272–285 (2013).
van Loosdregt, J. et al. Stabilization of the transcription factor Foxp3 by the deubiquitinase USP7 increases Treg-cell-suppressive capacity. Immunity 39, 259–271 (2013).
Dang, E. V. et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell 146, 772–784 (2011).
Fan, Y. et al. USP21 negatively regulates antiviral response by acting as a RIG-I deubiquitinase. J. Exp. Med. 211, 313–328 (2014).
Pannu, J. et al. Ubiquitin specific protease 21 is dispensable for normal development, hematopoiesis and lymphocyte differentiation. PLoS ONE 10, e0117304 (2015).
Zhang, J. et al. Identification of the E3 deubiquitinase ubiquitin-specific peptidase 21 (USP21) as a positive regulator of the transcription factor GATA3. J. Biol. Chem. 288, 9373–9382 (2013).
Cipolletta, D. et al. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 486, 549–553 (2012).
Dominguez-Villar, M., Baecher-Allan, C. M. & Hafler, D. A. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat. Med. 17, 673–675 (2011).
Shrestha, S. et al. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat. Immunol. 16, 178–187 (2015).
Huynh, A. et al. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat. Immunol. 16, 188–196 (2015).
Lee, J. H., Elly, C., Park, Y. & Liu, Y. C. E3 ubiquitin ligase VHL regulates hypoxia-inducible factor-1alpha to maintain regulatory T cell stability and suppressive capacity. Immunity 42, 1062–1074 (2015).
Zhao, Y. et al. E3 ubiquitin ligase Cbl-b regulates thymic-derived CD4+CD25+ regulatory T cell development by targeting Foxp3 for ubiquitination. J. Immunol. 194, 1639–1645 (2015).
Delgoffe, G. M. et al. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature 501, 252–256 (2013).
Joshi, N. S. et al. Regulatory T Cells in tumor-associated tertiary lymphoid structures suppress anti-tumor T cell responses. Immunity 43, 579–590 (2015).
Nishikawa, H. & Sakaguchi, S. Regulatory T cells in tumor immunity. Int. J. Cancer 127, 759–767 (2010).
Curiel, T. J. et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 10, 942–949 (2004).
Sato, E. et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl Acad. Sci. USA 102, 18538–18543 (2005).
Saito, T. et al. Two FOXP3CD4 T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat. Med. 22, 679–684 (2016).
Acknowledgements
Our research is supported by National Basic Research Program of China Grants 2014CB541803 and 2014CB541903; NSFC 30972702, 31170825, 31200647, 31200646, 81271835, 81270083, 81671611, 31670911, 31370863, 81330072; SMCST 14JC1406100; Shanghai Academic Research Leader 16XD1403800; Strategic Priority Research Program of the Chinese Academy of Sciences, Grant No. XDB19000000, XDB19040104; NIH-NSFC collaborative grant 81161120417; National Science and Technology Major Project 2012ZX10002007-003, 2013ZX10003009-002; NN-CAS Foundation. B.L. is a recipient of Shanghai ‘Rising Star’ program 10QA1407900, CAS ‘100-talent’ program and National Science Foundation for Distinguished Young Scholars 31525008. A.T. is a recipient of NSFC grant 31150110337. We thank R. Zhu, P. Wang, Y-J. Dang, F. Pan and members of Li laboratory for helpful discussion.
Author information
Authors and Affiliations
Contributions
Y.L. designed and performed experiments, and wrote the manuscript; Y.L. contributed to cellular experiments; S.W. and F.Z. contributed to the technical support; A.T. contributed to scientific discussion, writing and editing the manuscript; B.L. designed experiments, contributed to writing the manuscript and provided overall direction.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-8. (PDF 4084 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Li, Y., Lu, Y., Wang, S. et al. USP21 prevents the generation of T-helper-1-like Treg cells. Nat Commun 7, 13559 (2016). https://doi.org/10.1038/ncomms13559
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms13559
This article is cited by
-
FOXP3+ regulatory T cell perturbation mediated by the IFNγ-STAT1-IFITM3 feedback loop is essential for anti-tumor immunity
Nature Communications (2024)
-
Exploiting E3 ubiquitin ligases to reeducate the tumor microenvironment for cancer therapy
Experimental Hematology & Oncology (2023)
-
USP1-regulated reciprocal differentiation of Th17 cells and Treg cells by deubiquitinating and stabilizing TAZ
Cellular & Molecular Immunology (2023)
-
Treg plasticity and human diseases
Inflammation Research (2023)
-
Targeting ubiquitin signaling for cancer immunotherapy
Signal Transduction and Targeted Therapy (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.