Abstract
The ability to understand and control the electronic properties of individual molecules in a device environment is crucial for developing future technologies at the nanometre scale and below. Achieving this, however, requires the creation of three-terminal devices that allow single molecules to be both gated and imaged at the atomic scale. We have accomplished this by integrating a graphene field effect transistor with a scanning tunnelling microscope, thus allowing gate-controlled charging and spectroscopic interrogation of individual tetrafluoro-tetracyanoquinodimethane molecules. We observe a non-rigid shift in the molecule’s lowest unoccupied molecular orbital energy (relative to the Dirac point) as a function of gate voltage due to graphene polarization effects. Our results show that electron–electron interactions play an important role in how molecular energy levels align to the graphene Dirac point, and may significantly influence charge transport through individual molecules incorporated in graphene-based nanodevices.
Similar content being viewed by others
Introduction
Creating electronic devices based on single molecules is a key goal of modern nanotechnology1,2,3,4,5,6,7,8,9. Future progress in this area, however, hinges on developing a better understanding of the fundamental properties of individual molecules in new, complex electronic environments3,4,5,6,7,8,10,11,12. Molecules have been integrated into gated three-terminal electrical devices previously, allowing continuous tuning and characterization of molecular electronic properties2,3,4,5,6,7,8,10,11,13,14,15,16,17. Precise interpretations, however, have been made more difficult in these experiments by the fact that local chemical structures have not been well-characterized due to an inability to image individual molecules in device junctions. Single molecules with well-characterized chemical structure, on the other hand, are regularly studied via two-terminal scanning tunnelling microscopy (STM) techniques where electronic properties are not tunable via a third external gate electrode11,12,15,18,19,20,21 (as opposed to internal gating/doping generated by local impurity configurations12,20,22). Some progress has been made at introducing gate electrodes into scanned probe measurements of molecular systems23, but gate-tunable control of single-molecule charge states has not yet been demonstrated.
Here we combine STM and non-contact atomic force microscopy (nc-AFM) to demonstrate gate-tunable control of the charge state of individual, well-characterized tetrafluoro-tetracyanoquinodimethane (F4TCNQ) molecules at the surface of a graphene field effect transistor (FET) (F4TCNQ is a commonly used acceptor in molecular electronics). This system allows the substrate Fermi energy (EF) to be continuously tuned all the way through the lowest unoccupied molecular orbital (LUMO) energy of a single F4TCNQ molecule. Using STM spectroscopy we have determined the gate-dependent energetic evolution of the LUMO level (EL) and its associated vibronic modes relative to the graphene Dirac point (ED). We show that the energy alignment between EL and ED changes as the substrate charge carrier density is tuned by gating, indicating the presence of electron–electron interactions that renormalize the molecular quasiparticle energy. This is attributed to gate-tunable image-charge screening in graphene and is corroborated by ab initio calculations. Our findings reveal that such tunable electronic correlation effects significantly renormalize the electron addition and removal energies for individual molecules incorporated into graphene devices.
Results
Topographic imaging of anchored F4TCNQ molecules
The graphene FETs used here were made by transferring graphene grown via CVD techniques onto BN flakes supported by an SiO2 layer at the surface of a doped Si wafer (the doped Si provides the FET back-gate)23,24. Use of a BN substrate reduces charge inhomogeneity in graphene, allowing us to better probe intrinsic molecule/graphene electronic properties24. F4TCNQ (Fig. 1a) was selected for this study because its LUMO state has been predicted to lie close to the graphene Dirac point25, thus facilitating molecular charge state tunability. Scanning probe measurements of molecule-decorated devices were performed at T=5 K in ultra-high vacuum. Figure 1b shows an STM image of individual F4TCNQ molecules adsorbed onto the surface of a graphene/BN device at low coverage. F4TCNQ exhibits a dog-bone-like shape that resembles the LUMO of an isolated F4TCNQ molecule, similar to previous measurements of F4TCNQ molecules on graphene/metal26,27,28,29. Individual F4TCNQ molecules are not strongly pinned by the graphene/BN substrate, and so are prone to move quite easily when subjected to the local tip-induced electric fields required for high-resolution STM spectroscopy.
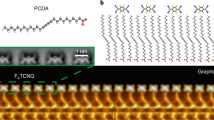
(a) Chemical structure of F4TCNQ and PCDA molecules. (b) STM image of F4TCNQ molecules decorating graphene/BN substrate (VS=2 V, It=5 pA). (c) Deposition of PCDA followed by deposition of F4TCNQ results in well-ordered PCDA islands with isolated F4TCNQ molecules adsorbed at the island edges (VS=2 V, It=10 pA). (d) F4TCNQ molecular adsorption geometry is shown with single-chemical-bond resolution via nc-AFM (qPlus) with a CO-functionalized tip. The nc-AFM image was taken in constant height mode at three different heights by lowering the tip at the two positions marked by blue arrows (320 pm (left) and 70 pm (right)). Hydrogen atoms can be resolved in the PCDA molecules, as well as triple bonds. F4TCNQ molecules are seen to adsorb with their nitrogen and fluorine atoms close to the terminal hydrogen atoms of PCDA, indicating hydrogen bonding as a likely source of F4TCNQ stabilization. The honeycomb lattice of graphene is clearly resolved. (All images taken at T=5 K).
To overcome this problem, we devised an anchoring strategy to immobilize individual F4TCNQ molecules by using electronically inert 10,12-pentacosadiynoic acid (PCDA) as a molecular anchor (Fig. 1a). We evaporated PCDA molecules onto graphene/BN just before deposition of F4TCNQ molecules. Our STM images reveal that PCDA molecules self-assemble into ordered islands on graphene/BN that exhibit straight edges (Fig. 1c), consistent with previously reported behaviour of PCDA on graphene/SiC (ref. 30). Individual F4TCNQ molecules anchor nicely to the edge of PCDA islands, as seen in Fig. 1c. These edge-anchored F4TCNQ molecules were sufficiently stable for high-resolution STS and nc-AFM measurements. Nc-AFM with a CO tip was used to determine the precise adsorption geometry of F4TCNQ on the PCDA-functionalized graphene/BN surface, as shown in Fig. 1d. In such images contrast is caused by short-range chemical forces, and bright areas exhibiting high frequency shift tend to represent surface regions with higher electron density (such as atoms and chemical bonds31,32,33,34,35). The nc-AFM image in Fig. 1d thus reveals the ‘wire-frame’ chemical structure and adsorption geometry of F4TCNQ molecules attached to PCDA molecular anchors. Our results show that F4TCNQ molecules align along the armchair direction of graphene and that there is no significant chemical interaction between F4TCNQ and PCDA (the faint lines seen connecting the F4TCNQ and PCDA molecules are a common feature for adsorbates bound by weak hydrogen and van der Waals interaction)35,36,37,38.
Electronic structure of F4TCNQ on graphene
We measured the electronic structure of individual F4TCNQ molecules anchored to PCDA on our graphene/BN devices through the use of STM spectroscopy (the F4TCNQ electronic structure was not significantly affected by the nearby PCDA (Supplementary Fig. 1)). The inset in Fig. 2a shows a typical dI/dV spectrum measured over the range −0.4 V<VS<0.4 V (VS is the sample voltage with respect to the tip) with the back-gate held at VG=0 V. Spectroscopy was acquired on the outer edges of the molecule to avoid inelastic tunnelling effects39 (Supplementary Fig. 2). Two broad peaks are visible in the VS>0 V range, marked L and L′. These peaks are asymmetric, as seen in the adjacent high-resolution dI/dV spectrum in Fig. 2a. For VS<0 V only one peak is seen, marked ‘charging’, which is significantly sharper than the VS>0 V peaks21,40,41. The L peak (VS>0) is derived from the F4TCNQ LUMO state. The charging peak (VS<0), on the other hand, does not directly indicate a feature in the molecular density of states but rather occurs due to tip-induced band bending as the tip electric field pulls the LUMO state below the Fermi energy (VS=0 V), causing it to fill with charge21,40,41,42,43. We attribute the asymmetric structure of the L and L′ peaks to vibronic sidebands, as has been observed in other systems23,39,44,45. To extract the experimental energies of the vibrations involved, we fit the F4TCNQ spectrum to a sum of Gaussian peaks. The L feature is fit well by a peak located at EL=61±6 meV (blue dashed line in Fig. 2a) along with five other satellite peaks evenly spaced every 37±7 meV (orange dashed lines). The L′ feature is similarly fit well by a peak located at EL′=288±23 meV (purple dashed line) with three additional satellite peaks evenly spaced every 37±7 meV (orange dashed lines) (see Supplementary Fig. 3 for details of fit).
(a) dI/dV spectrum for a single F4TCNQ molecule on graphene/BN shows two main peaks spaced by ∼227 meV for VS>0 V, and one peak for VS<0 V. Peaks for VS>0 V originate from LUMO and vibronic modes while the peak at VS<0 V originates from tip-induced charging of the LUMO level. Initial tunnelling parameters: It=30 pA , VS=0.4 V, VAC=8 mV. (b) Ab initio calculated energies of phonon modes for F4TCNQ/graphene (grey), as well as electron-phonon coupling strength between phonon modes and LUMO state (green curve, broadened by a 12 meV full-width Gaussian). The phonons with highest electron-phonon coupling occur at ω1∼34 meV and ω2∼183 meV. (c) dI/dV spectrum of F4TCNQ/graphene/BN for VG=−50 V (blue) shows F4TCNQ vibronic states for a neutral molecule (LUMO0). dI/dV spectrum of the same molecule at VG=30 V (red) shows that vibronic states for a charged molecule (LUMO−) switch their energy alignment from increasing energy ordering to decreasing energy ordering when the charge state is switched by the gate (the portion of the blue (red) curve below (above) EF has been scaled by 0.4 (0.2) to fit on the plot). Initial tunneling parameters: It=15 pA , VS=1 V, VAC=12 mV.
To test our hypothesis regarding the vibrational origin and structure of the L and L′ peaks, we calculated the hybridized molecular orbitals and vibrational modes of an F4TCNQ molecule on graphene, as well as the associated electron–phonon coupling, from first principles. As shown in Fig. 2b, numerous vibrational modes exist in the range 0<E<300 meV. Calculation of the electron-phonon coupling for each of these modes, however, (Fig. 2b, green curve) shows that the strongest coupling occurs at ω1≈34 meV and ω2≈183 meV (Supplementary Note 1). These energies correspond to a uniaxial stretching mode with Ag symmetry (ω1=34 meV) and a breathing mode of the inner carbon ring also having Ag symmetry (ω2=183 meV). The calculated energies agree reasonably well with the experimental energy spacing of the peaks extracted from within the L and L′ features of Fig. 2a (37±7 meV), as well as the energy difference EL′–EL=227±24 meV. This shows that L′ is a vibronic satellite of L due to phonons having energy ω2 and that the internal structure of both L and L′ represents vibronic satellites due to phonons having energy ω1. A more detailed analysis involving a cumulant expansion to calculate the spectral function including vibronic modes also agrees with our measured spectra, supporting the vibronic interpretation of the spectral lineshape (Supplementary Note 1).
Reversible charge state switching of molecules using a back-gate
A unique aspect of this study is that we are able to reversibly control the charge state of a single F4TCNQ molecule by continuously tuning the substrate Fermi level past the LUMO energy level via application of an electrostatic back-gate. Figure 2c shows STM dI/dV spectra of a single anchored F4TCNQ molecule at two different back-gate voltages (VG). Here we label the empty and occupied LUMO orbital as LUMO0 and LUMO−, respectively. The blue curve acquired at VG=−50 V shows the LUMO level well above EF and thus empty (LUMO0), resulting in a neutral molecule at this gate voltage. In contrast, the red dI/dV trace acquired at VG=+30 V shows the LUMO well below EF and thus filled by an electron, causing the molecule to become negatively charged (LUMO−). A notable difference between the red and blue curves is that the vibrational sidebands for the LUMO− state extend downwards to more negative VS values compared with the sidebands for LUMO0 which extend upwards to more positive VS values. This can be explained by the fact that higher-energy electron-like vibronic excitations (for LUMO0) occur at higher values of VS, whereas higher-energy hole-like vibronic excitations (for LUMO−) occur at lower values of VS. Our first-principles calculation of the spectral function using a cumulant expansion46 confirms this intuitive electron–hole symmetry and reproduces the observed vibronic spectra (Supplementary Fig. 4).
Non-rigid shift of LUMO energy
Our ability to gate the substrate of an adsorbed molecule allows us to address the fundamental issue of the importance of electron–electron interactions for molecule/substrate systems. We do this by measuring how the energy level of the molecular orbital changes relative to the graphene band structure as EF is swept by the gate. In the absence of electron–electron interactions we expect the position of the molecular level relative to ED to be independent of EF. If, on the other hand, electron–electron interactions play a significant role then we expect the LUMO energy (EL) to shift relative to ED as EF is tuned. We measured this effect by acquiring dI/dV spectra both on a single F4TCNQ molecule (to obtain EL) and off the molecule (to obtain ED) as a function of gate voltage. Figure 3a shows a plot of the resulting ‘on molecule’ dI/dV spectra as a function of gate voltage (−40 V<VG<+40 V), while Fig. 3b shows a plot of the ‘off molecule’ spectra (that is, for bare graphene). The on-molecule spectra show the molecular LUMO level continuously sweeping from an empty orbital state (LUMO0) for VG<0 V to a filled orbital state (LUMO−) for VG>0 V. Similarly, the off-molecule spectra show the graphene Dirac point sweeping from above EF for VG<0 V(the p-doped regime) to below EF for VG>0 V(the n-doped regime). To extract the experimental gate-dependent values of EL, we fit the dI/dV spectra in Fig. 3a with sums of Gaussians. The resulting LUMO energies are shown in Fig. 3a at the red dot locations. The gate-dependent ED values were obtained from the spectra of Fig. 3b by fitting inverted Gaussians to the prominent local minimum of each spectrum (resulting in the black dots shown in Fig. 3b).
(a) dI/dV spectra obtained with STM tip held over a single F4TCNQ molecule recorded at different gate voltages show that the LUMO state and vibronic sidebands shift relative to EF as the gate is swept. Red dots mark energy locations of the LUMO state at different gate voltages, extracted by fitting a sum of Gaussian peaks to the dI/dV spectra (initial tunneling parameters: It=15 pA , VS=1 V, VAC=12 mV). (b) dI/dV spectra obtained with the STM tip held over a bare patch of graphene/BN near an F4TCNQ molecule (distance=4 nm) recorded at different gate voltages show dependence of Dirac point energy (ED) on gate voltage. Black dots mark Dirac point obtained by fitting inverted Gaussians to the minimum of each spectrum. Final ED values are obtained by subtracting the inelastic phonon energy of 63 meV from these measured features54,55 (initial tunneling parameters: It=60 pA, VS=0.5 V, VAC=12 mV).
Figure 4 shows a direct comparison of the experimental ED and EL values as a function of gate voltage (because ED is difficult to obtain for some gate voltages we use values here that are obtained by fitting a characteristic square root function to the data of Fig. 3b (Supplementary Fig. 5)). For small gate voltage (low charge carrier density) ED and EL are seen to lie almost directly on top of each other. When the gate voltage magnitude is increased (resulting in higher charge carrier density) however, the values diverge, separating by as much as 100 meV at the highest gate voltage (corresponding to a charge carrier density of ∼3 × 1012 cm−2 at VG=60 V). The energy difference between ED and EL (ED–EL) is seen to increase monotonically with increasing charge carrier density for both electrons and holes, suggesting that electron–electron interactions play a role in determining EL for this adsorbate system. The observation that ED–EL does not depend on the polarity of graphene charge carriers rules out simple band bending as an explanation for the energy shift, since band bending would shift EL to higher energies when EL–EF>0, opposite to what is observed21,40,47.
The experimental LUMO energies (red dots) and the theoretically predicted ones (blue curve) agree qualitatively by lying below the Dirac point energy (dashed line, see Supplementary Fig. 5 for fitting details) for all gate voltages away from charge neutrality. The experimental error in the LUMO energy is estimated as the mean half-width of the Gaussian fits to the F4TCNQ spectra (16 meV). The experimental error in ED is estimated using the fit of the measured ED to the square root dependence of ED versus VG (the r.m.s. of the fit residuals=19 meV). Inset: theoretical energy renormalization of the LUMO level due to interaction of LUMO charge with induced graphene image charge as a function of EF.
Discussion
We are able to explain this behaviour as the result of Coulomb interaction between charge added to an F4TCNQ molecular orbital (via tunnelling), and the electronic polarization that it induces in the graphene substrate. This many-electron interaction always lowers the energy of a system, since the interaction between charge added to an adsorbate and its image charge in the substrate is always attractive. Such effects are well known to reduce the energy gap between affinity and ionization levels for adsorbates on conventional substrates13,48,49,50. For an adsorbate on graphene this effect is expected to be tunable since polarizability depends on the density of states at EF, which is readily changed by gating a graphene device.
To estimate the expected magnitude of this effect for comparison to our data, we calculated how the F4TCNQ LUMO energy level is renormalized via screening within many-body perturbation theory (standard DFT treatments of the Kohn–Sham eigenvalues do not take this non-local effect into account). This was accomplished by modelling an electron that has tunnelled into the empty LUMO level as a point charge located a distance z* above the graphene plane (z* is estimated to be 3 Å from our ab initio calculations of F4TCNQ/graphene, in reasonable agreement with the tip-height change during AFM measurements (Fig. 1d)). The point charge exposes the graphene substrate to a Coulomb potential, ϕext(r), which induces screening charge density, nind(r), in the graphene. We calculated nind(r) and the change it causes to the electrostatic potential, ϕind(r), within linear response theory using the RPA dielectric function of graphene53 (the BN substrate was taken into account by choosing a background dielectric constant of  , see Supplementary Notes 2 and 3). The screening-induced lowering of the LUMO energy, ΔEL, was estimated by evaluating ϕind(r) at the location of the point charge above the graphene surface thus yielding
, see Supplementary Notes 2 and 3). The screening-induced lowering of the LUMO energy, ΔEL, was estimated by evaluating ϕind(r) at the location of the point charge above the graphene surface thus yielding  (ref. 48). The inset in Fig. 4 shows the calculated ΔEL values as a function of EF. For EF=ED the energy correction is smallest since graphene has no density of states at the Fermi level when EF is aligned to the Dirac point. As EF shifts away from ED, the energy correction increases equally for both electron and hole-doping since the carrier RPA dielectric function for graphene is electron–hole symmetric. The near-linear dependence of ΔEL on EF stems from the linear graphene density of states, ρ(E), and the fact that the graphene electronic susceptibility is proportional to ρ(EF).
(ref. 48). The inset in Fig. 4 shows the calculated ΔEL values as a function of EF. For EF=ED the energy correction is smallest since graphene has no density of states at the Fermi level when EF is aligned to the Dirac point. As EF shifts away from ED, the energy correction increases equally for both electron and hole-doping since the carrier RPA dielectric function for graphene is electron–hole symmetric. The near-linear dependence of ΔEL on EF stems from the linear graphene density of states, ρ(E), and the fact that the graphene electronic susceptibility is proportional to ρ(EF).
To compare the calculated ΔEL to spectroscopy taken on individual F4TCNQ molecules, we can add it to our gate-dependent measurements of ED (taking into account a small offset constant observed experimentally at charge neutrality). In the absence of any screening effects (ΔEL=0 eV), this would result in EL≈ED at every gate voltage (the simple ‘rigid shift’ case). The Coulomb-induced renormalization effects described above, however, cause this procedure to result in a nontrivial shift of EL with respect to ED as a function of gate voltage. Figure 4 shows a plot of the resulting renormalized EL value (blue curve) as a function of gate voltage compared with the experimental EL values (red dots). For small gate voltages the renormalized EL value coincides with ED, but as the gate voltage (and carrier density) magnitude is raised the calculated EL values fall increasingly downward compared with ED, just as seen for the experimental EL values. The model thus captures both the observed independence of the LUMO renormalization on the graphene carrier type (electrons or holes) and also reproduces the general magnitude of the molecular orbital energy change. The match is not perfect (the experimental drop in EL tends to be larger than the calculated ΔEL), but is reasonable considering that the calculation has no adjustable parameters. One possible source for the discrepancy between theory and experiment are intra-molecular electron–electron interactions, which are not accounted for in the simple image charge model. We have examined the effect of these interactions on the LUMO energy using an Anderson model approach and find that they do lead to additional LUMO energy renormalization, but smaller in magnitude than the image charge effects (Supplementary Note 4).
In conclusion, we demonstrate reversible tuning of the charge state of individual F4TCNQ molecules using an electrostatically back-gated graphene device. The molecular adsorption geometry is imaged via nc-AFM with single-chemical-bond resolution and the gate-dependent molecular electronic structure is determined via STM spectroscopy. We find that molecular vibronic modes can be switched from electron-like energy alignment to hole-like energy alignment depending on the tunable molecular charge state, in agreement with cumulant-expansion theory. We additionally observe a non-rigid shift in the LUMO energy relative to the Dirac point as a function of gate voltage that can be explained by many-electron interaction renormalization of the LUMO energy caused by tunable substrate polarization effects.
Methods
Graphene device fabrication
A back-gated graphene/BN/SiO2 device was prepared by overlaying CVD-grown graphene onto hexagonal boron nitride (h-BN) flakes exfoliated onto a SiO2/Si substrate. h-BN flakes were exfoliated onto heavily doped silicon wafers and annealed at 500 °C for several hours in air prior to graphene transfer. The graphene was grown on copper foil by the CVD method51 and transferred to the BN/SiO2 substrate via a poly methyl methacrylate stamp52. Electrical contact was made to the graphene by depositing Ti (10 nm thick)/Au (30 nm thick) electrodes using the stencil mask technique.
Sample preparation
The graphene device was first annealed in flowing Ar/H2 gas at 350 °C and then annealed subsequently in UHV at T∼350 °C for several hours until an atomically clean surface was achieved before the deposition of molecules. PCDA and F4TCNQ were deposited consecutively onto the clean graphene substrate at room temperature using Knudsen cell evaporators in the UHV chamber.
STM/AFM measurements
STM/nc-AFM measurements were performed using a qPlus-equipped commercial Omicron LT-STM/AFM under UHV conditions at T=5 K using tungsten tips. STM topography was obtained in constant-current mode. STM tips were calibrated on a Au(111) surface by measuring the Au(111) Shockley surface state before all STS measurements. STS was performed under open feedback conditions by lock-in detection of an alternating current tunnel current with a bias modulation of 6–16 mV (r.m.s.) at 400 Hz added to the tunnelling bias. The tips were functionalized for nc-AFM imaging by picking up individual CO molecules on a Au(111) surface33. nc-AFM images were recorded by measuring the frequency shift of a qPlus resonator, while scanning over the molecule in constant-height mode (f0=28.7 kHz, Q=90 k, A=60 pm). WSxM software was used to process all STM/nc-AFM images53.
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
Additional information
How to cite this article: Wickenburg, S. et al. Tuning charge and correlation effects for a single molecule on a graphene device. Nat. Commun. 7, 13553 doi: 10.1038/ncomms13553 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Aviram, A. & Ratner, M. A. Molecular rectifiers. Chem. Phys. Lett. 29, 277–283 (1974).
Tao, N. J. Electron transport in molecular junctions. Nat. Nano 1, 173–181 (2006).
Sun, L. et al. Single-molecule electronics: from chemical design to functional devices. Chem. Soc. Rev. 43, 7378–7411 (2014).
Aradhya, S. V. & Venkataraman, L. Single-molecule junctions beyond electronic transport. Nat. Nano 8, 399–410 (2013).
McCreery, R. L. & Bergren, A. J. Progress with molecular electronic junctions: meeting experimental challenges in design and fabrication. Adv. Mater. 21, 4303–4322 (2009).
Perrin, M. L., Burzuri, E. & van der Zant, H. S. J. Single-molecule transistors. Chem. Soc. Rev. 44, 902–919 (2015).
Moth-Poulsen, K. & Bjornholm, T. Molecular electronics with single molecules in solid-state devices. Nat. Nano 4, 551–556 (2009).
Joachim, C., Gimzewski, J. K. & Aviram, A. Electronics using hybrid-molecular and mono-molecular devices. Nature 408, 541–548 (2000).
Jia, C. et al. Covalently bonded single-molecule junctions with stable and reversible photoswitched conductivity. Science 352, 1443–1445 (2016).
Liang, W., Shores, M. P., Bockrath, M., Long, J. R. & Park, H. Kondo resonance in a single-molecule transistor. Nature 417, 725–729 (2002).
Haiss, W. et al. Precision control of single-molecule electrical junctions. Nat. Mater. 5, 995–1002 (2006).
Martinez-Blanco, J. et al. Gating a single-molecule transistor with individual atoms. Nat. Phys. 11, 640–644 (2015).
Perrin, M. L. et al. Large tunable image-charge effects in single-molecule junctions. Nat. Nano 8, 282–287 (2013).
Reed, M. A., Zhou, C., Muller, C. J., Burgin, T. P. & Tour, J. M. Conductance of a molecular junction. Science 278, 252–254 (1997).
Xu, B. & Tao, N. J. Measurement of single-molecule resistance by repeated formation of molecular junctions. Science 301, 1221–1223 (2003).
Park, H. et al. Nanomechanical oscillations in a single-C60 transistor. Nature 407, 57–60 (2000).
Song, H. et al. Observation of molecular orbital gating. Nature 462, 1039–1043 (2009).
Schull, G., Frederiksen, T., Arnau, A., Sanchez-Portal, D. & Berndt, R. Atomic-scale engineering of electrodes for single-molecule contacts. Nat. Nano 6, 23–27 (2011).
Cui, X. D. et al. Reproducible measurement of single-molecule conductivity. Science 294, 571–574 (2001).
Yamachika, R., Grobis, M., Wachowiak, A. & Crommie, M. F. Controlled atomic doping of a single C60 molecule. Science 304, 281–284 (2004).
Fernández-Torrente, I., Kreikemeyer-Lorenzo, D., Stróżecka, A., Franke, K. J. & Pascual, J. I. Gating the charge state of single molecules by local electric fields. Phys. Rev. Lett. 108, 036801 (2012).
Lee, D. H. & Gupta, J. A. Tunable field control over the binding energy of single dopants by a charged vacancy in GaAs. Science 330, 1807–1810 (2010).
Riss, A. et al. Imaging and tuning molecular levels at the surface of a gated graphene device. ACS Nano 8, 5395–5401 (2014).
Decker, R. et al. Local electronic properties of graphene on a BN substrate via scanning tunneling microscopy. Nano Lett. 11, 2291–2295 (2011).
Pinto, H., Jones, R., Goss, J. P. & Briddon, P. R. p-type doping of graphene with F4-TCNQ. J. Phys.: Condens. Mater. 21, 402001 (2009).
Stradi, D. et al. Controlling the spatial arrangement of organic magnetic anions adsorbed on epitaxial graphene on Ru(0001). Nanoscale 6, 15271–15279 (2014).
Barja, S. et al. Self-organization of electron acceptor molecules on graphene. Chem. Commun. 46, 8198–8200 (2010).
Chi, M. & Zhao, Y.-P. First principle study of the interaction and charge transfer between graphene and organic molecules. Comput. Mater. Sci. 56, 79–84 (2012).
Sun, J. T., Lu, Y. H., Chen, W., Feng, Y. P. & Wee, A. T. S. Linear tuning of charge carriers in graphene by organic molecules and charge-transfer complexes. Phys. Rev. B 81, 155403 (2010).
Alaboson, J. M. P. et al. Templating sub-10 nm atomic layer deposited oxide nanostructures on graphene via one-dimensional organic self-assembled monolayers. Nano Lett. 13, 5763–5770 (2013).
de Oteyza, D. G. et al. Direct imaging of covalent bond structure in single-molecule chemical reactions. Science 340, 1434–1437 (2013).
Emmrich, M. et al. Subatomic resolution force microscopy reveals internal structure and adsorption sites of small iron clusters. Science 348, 308–311 (2015).
Gross, L., Mohn, F., Moll, N., Liljeroth, P. & Meyer, G. The chemical structure of a molecule resolved by atomic force microscopy. Science 325, 1110–1114 (2009).
Gross, L. et al. Organic structure determination using atomic-resolution scanning probe microscopy. Nat. Chem. 2, 821–825 (2010).
Gross, L. et al. Bond-order discrimination by atomic force microscopy. Science 337, 1326–1329 (2012).
Hapala, P. et al. Mechanism of high-resolution STM/AFM imaging with functionalized tips. Phys. Rev. B 90, 085421 (2014).
Hämäläinen, S. K. et al. Intermolecular contrast in atomic force microscopy images without intermolecular bonds. Phys. Rev. Lett. 113, 186102 (2014).
Zhang, J. et al. Real-space identification of intermolecular bonding with atomic force microscopy. Science 342, 611–614 (2013).
Pavliček, N., Swart, I., Niedenführ, J., Meyer, G. & Repp, J. Symmetry dependence of vibration-assisted tunneling. Phys. Rev. Lett. 110, 136101 (2013).
Brar, V. W. et al. Gate-controlled ionization and screening of cobalt adatoms on a graphene surface. Nat. Phys. 7, 43–47 (2011).
Wang, Y. et al. Mapping Dirac quasiparticles near a single Coulomb impurity on graphene. Nat. Phys. 8, 653–657 (2012).
Marczinowski, F., Wiebe, J., Meier, F., Hashimoto, K. & Wiesendanger, R. Effect of charge manipulation on scanning tunneling spectra of single Mn acceptors in InAs. Phys. Rev. B 77, 115318 (2008).
Nazin, G. V., Qiu, X. H. & Ho, W. Charging and interaction of individual impurities in a monolayer organic crystal. Phys. Rev. Lett. 95, 166103 (2005).
Wang, S., Wang, W., Hong, Y., Tang, B. Z. & Lin, N. Vibronic state assisted resonant transport in molecules strongly anchored at an electrode. Phys. Rev. B 83, 115431 (2011).
Qiu, X. H., Nazin, G. V. & Ho, W. Vibronic states in single molecule electron transport. Phys. Rev. Lett. 92, 206102 (2004).
Story, S. M., Kas, J. J., Vila, F. D., Verstraete, M. J. & Rehr, J. J. Cumulant expansion for phonon contributions to the electron spectral function. Phys. Rev. B 90, 195135 (2014).
Wu, S. W., Nazin, G. V., Chen, X., Qiu, X. H. & Ho, W. Control of relative tunneling rates in single molecule bipolar electron transport. Phys. Rev. Lett. 93, 236802 (2004).
Neaton, J. B., Hybertsen, M. S. & Louie, S. G. Renormalization of molecular electronic levels at metal–molecule interfaces. Phys. Rev. Lett. 97, 216405 (2006).
Torrente, I. F., Franke, K. J. & Pascual, J. I. Spectroscopy of C 60 single molecules: the role of screening on energy level alignment. J. Phys.: Condens. Mater. 20, 184001 (2008).
Kubatkin, S. et al. Single-electron transistor of a single organic molecule with access to several redox states. Nature 425, 698–701 (2003).
Li, X. et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 324, 1312–1314 (2009).
Reina, A. et al. Transferring and identification of single- and few-layer graphene on arbitrary substrates. J. Phys. Chem. C 112, 17741–17744 (2008).
Horcas, I. et al. WSXM: a software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 78, 013705 (2007).
Brar, V. W. et al. Observation of carrier-density-dependent many-body effects in graphene via tunneling spectroscopy. Phys. Rev. Lett. 104, 036805 (2010).
Zhang, Y. et al. Giant phonon-induced conductance in scanning tunnelling spectroscopy of gate-tunable graphene. Nat. Phys. 4, 627–630 (2008).
Acknowledgements
This research was supported by the Director, Office of Science, Office of Basic Energy Sciences, Materials Sciences and Engineering Division, of the US Department of Energy under contract no. DE-AC02-05CH11231 (Nanomachine program-KC1203) (STM imaging and spectroscopy, cumulant-expansion studies of spectral line shapes and phonon sidebands), Molecular Foundry (graphene growth, growth characterization), National Science Foundation grant DMR-1206512 (sample fabrication) and National Science Foundation grant DRM-1508412 (electron-phonon coupling calculations). K.W. and T.T. acknowledge support from the Elemental Strategy Initiative conducted by the MEXT, Japan and a Grant-in-Aid for Scientific Research on Innovative Areas ‘Science of Atomic Layers’ from JSPS. J. Lu and A.H.C.N. acknowledge the support from the National Research Foundation, Prime Minister Office, Singapore, under its Medium Sized Centre Programme and CRP award no. R-144-000-295-281. J. Lischner acknowledges support from EPSRC under grant no. EP/N005244/1 (development of image charge model). Computational resources have been provided by DOE at NERSC. A.R. acknowledges fellowship support by the Austrian Science Fund (FWF): J3026-N16.
Author information
Authors and Affiliations
Contributions
S.W., J. Lu, H.-Z.T. and A.O. designed and performed experiments, analysed data and wrote the paper. J. Lischner conceived of and performed theoretical calculations, and wrote the paper. C.K. performed Anderson model calculations and gave conceptual advice. A.R. designed the experiments, gave technical support and conceptual advice, and edited the manuscript. A.B., D.W. gave technical support and conceptual advice and helped with the experiments. H.S.J. and R.K. facilitated the sample fabrication and gave technical support. K.W. and T.T. gave technical support and grew h-BN for the device. S.G.L supervised the theoretical calculations. S.G.L, A.H.C.N., A.Z. and M.F.C. coordinated the collaboration. M.F.C. designed and supervised the experiments, supervised the data analysis and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-11, Supplementary Notes 1-4 and Supplementary References (PDF 11202 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wickenburg, S., Lu, J., Lischner, J. et al. Tuning charge and correlation effects for a single molecule on a graphene device. Nat Commun 7, 13553 (2016). https://doi.org/10.1038/ncomms13553
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms13553
This article is cited by
-
A molecular shift register made using tunable charge patterns in one-dimensional molecular arrays on graphene
Nature Electronics (2020)
-
A shift in molecular devices
Nature Electronics (2020)
-
Frustrated supercritical collapse in tunable charge arrays on graphene
Nature Communications (2019)
-
Selective control of molecule charge state on graphene using tip-induced electric field and nitrogen doping
npj 2D Materials and Applications (2019)
-
Noisy defects in the high-Tc superconductor Bi2Sr2CaCu2O8+x
Nature Communications (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.