Abstract
Locomotion is one of the major energetic costs faced by animals and various strategies have evolved to reduce its cost. Birds use interspersed periods of flapping and gliding to reduce the mechanical requirements of level flight while undergoing cyclical changes in flight altitude, known as undulating flight. Here we equipped free-ranging marine vertebrates with accelerometers and demonstrate that gait patterns resembling undulating flight occur in four marine vertebrate species comprising sharks and pinnipeds. Both sharks and pinnipeds display intermittent gliding interspersed with powered locomotion. We suggest, that the convergent use of similar gait patterns by distinct groups of animals points to universal physical and physiological principles that operate beyond taxonomic limits and shape common solutions to increase energetic efficiency. Energetically expensive large-scale migrations performed by many vertebrates provide common selection pressure for efficient locomotion, with potential for the convergence of locomotory strategies by a wide variety of species.
Similar content being viewed by others
Introduction
Animals use diverse strategies to reduce the energetically expensive cost of locomotion1 that range from morphological solutions, such as compliant tendons2 and low drag-coefficients3, to behavioural solutions, such as porpoising4, selective use of currents and winds5 or travelling in formation6,7. One such behavioural strategy, intermittent locomotion, is widely used by both vertebrates and invertebrates to reduce the cost of movement and includes terrestrial, avian and aquatic fauna8. Birds in particular use various types of intermittent locomotion to reduce the mechanical power required for horizontal travel9,10,11. Undulating flight is a special form of intermittent locomotion and involves gliding with flexed wings interspersed with active flapping. Potential energy from gravity and altitude is translated into horizontal distance via gliding, which is thought to result in savings of mechanical power compared with continuous level flight10,11,12. Although only described for flying birds and bats, undulating flight could theoretically occur in any fluid environment and reduce the cost of locomotion under the right physical conditions9.
Recent work using animal-attached tags has revealed that a range of air-breathing marine animals glide during phases of ascents or descents while assisted by buoyancy, which has been attributed to increase dive efficiency through economic transiting from surface to depth13,14,15,16. Here we show that intermittent glides of four species of marine vertebrates are analogous to undulating flight as observed in flying animals. A direct comparison of continuous and intermittently swimming seals revealed that during intermittent swimming, less locomotory effort was performed than during continuous swimming at corresponding velocities.
Results
Undulating flight in water
Animal-attached electronic tags logged acceleration and depth in two species of sharks (whale shark, Rhincodon typus, and white shark, Carcharodon carcharias, Table 1) and two species of pinnipeds (northern fur seal, Callorhinus ursinus, and southern elephant seal, Mirounga leonina, Table 1). The results reveal that their movement patterns show a high degree of similarity (also see Kawabe et al.17). All four species performed undulating flight, where passive gliding descents are interspersed with ascents characteristic of active propulsion (Fig. 1; Supplementary Movie 1). All individuals of the four species exhibited variability in the locomotory power output, the duty factor (the percentage of time spent in active locomotion compared with gliding18), as well as the sink- and climb-rate (Table 2). Elephant seals swam almost exclusively continuously at depths shallower than ∼15 m, whereas at deeper depths they adopted intermittent locomotion (Figs 2 and 3). Moreover, duty factor decreased with increasing depth in fur seals (Spearman's ρ=−0.244, P<0.01, n=126), whereas there was no detectable change in whale sharks (Spearman's ρ=0.089, P=0.120, n=308).
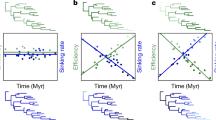
Undulating flight is characterized by altering passive descent gliding and active climbing. (a) Schematic figure of a starling engaged in intermittent flight, adapted from Rayner et al.10 Similar patterns are observed in elephant seals (b) and northern fur seals (d) during the bottom phase of dives, white sharks (c) and whale sharks (e) throughout the water-column. Patterns are consistent irrespective of medium (air (a) versus water (b, c, d, e)) and mode of propulsion (pectoral propulsion (a, d), versus caudal propulsion (b, c, e)).
Observed gait patterns in relation to buoyancy conditions experienced by elephant seals. Changes in buoyancy with depth were calculated for an elephant seal based on body composition before migration following the moult (Methods). During the bottom phase of u-shaped dives, elephant seal 1 (triangles) and elephant seal 2 (circles) swam continuously (red) at shallow (<∼15 m) depth and started to adopt undulating flight (blue) at deeper (>∼15 m) depths, where their negative buoyancy was greater.
Changes in gait associated with the two swimming patterns in a free-swimming southern elephant seal (M. leonina); continuous locomotion (red) at shallow depth with a velocity of 2 m s−1 (a) and 3.5 m s−1 (c) and undulating flight (purple) at the 2 m s−1 (b) and 3 m s−1 (d). Power requirements (ascertained via ODBA), (e) increased significantly with swimming-speed in both continuous (y=0.02x2–0.06x–0.13; r2=0.78; F2,31=56.38; P<0.0001 ) and undulating flight corrected for horizontal speed (y=0.004x2 +0.007x+0.050; r2=0.74; F2,75=110.10; P<0.0001). 95% confidence intervals are displayed.
Kinematics and power requirements in continuous and intermittent locomotion
The elephant seal carrying speed and acceleration sensors showed significant increases in the duty factor as it increased its swimming speed (cf. Fig. 3; y=0.94x+0.33; r2=0.36; F1,76=42.43, P<0.0001). Detailed analysis of locomotory effort through the activity metric overall dynamic body acceleration (ODBA)19,20, which integrates estimated metabolic effort for movement over limb-stroke frequency and amplitude21, revealed this elephant seal utilized a smaller range of locomotory power output in 'undulating locomotion' (1st and 3rd quartile of measured ODBA: 0.12 g and 0.14 g, respectively) compared with continuous locomotion (1st and 3rd quartile of measured ODBA: 0.09 g and 0.15 g, respectively). A direct comparison of undulating flight and continuous locomotion at corresponding velocities revealed that the elephant seal engaged in less locomotory power output (as ascertained by ODBA) while swimming intermittently (Fig. 3). Assuming an underlying linear relationship of ODBA and oxygen consumption19,20,22, the elephant seal saved between 13% and 35.6% of activity-related metabolism by utilizing intermittent locomotion at depth greater than ∼15 m compared with continuous locomotion at shallower depths (Fig. 3). At routine horizontal travel speeds of <2.5 m s−1, ODBA of undulating swimming (0.081±0.015 g) was significantly lower (Mann−Whitney-U test, U(69)=226, Z=−2.881, P<0.01) compared with continuous swimming (0.093±0.010 g), representing a mean saving of ∼13%.
Discussion
Our results show that intermittent locomotion, analogous to undulating flight, is found in a range of marine species, such as sharks and seals (Fig. 1; also see Kawabe et al.17).This high degree of similarity in the movement patterns of the different species is surprising considering their distinct evolutionary history and differing modes of propulsion; fur seals swim by pectoral propulsion, elephant seals use modified hind limbs as flippers and sharks propel themselves using a caudal fin. The intermittent locomotion observed was associated with changes in depth, thus resembling undulating flight described in birds (Fig. 1; Supplementary Movie 1).
Mechanical models have shown that modest to significant savings of metabolic power can be achieved by undulating flight in both air and water9,10,23. Most aquatic studies have focused on the metabolic savings that are achieved while animals are assisted by buoyancy13,14,15, thus losing potential energy that has to be repaid (but see Davis & Weihs16). Indeed, such studies have focused on locomotory adjustments to changing buoyant forces as pulmonary and fur associated air is compressed with increasing depth, resulting in less stroking and more gliding. In contrast, our study shows that intermittent locomotion is also an effective mode of locomotion when the net change in potential energy is zero, that is, no net change of depth (Figs 1 and 3) and buoyancy remains near constant, as the amplitude of individual undulations is too small to elicit a significant change in the volume of respiratory and fur-associated air.
As ODBA has been shown to correlate linearly with oxygen consumption in the exercising marine and terrestrial animals examined thus far19,20,22, our treatise demonstrates that muscular work is more pronounced in continuous locomotion than in intermittent locomotion. The kinematics of undulating flight vary according to travel speed and morphology in birds11 and we observed a similar relationship in our marine vertebrates (Table 2; Fig. 3). Indeed, similar to the elephant seal in this study (Fig. 3), birds performing undulating flight have been shown to increase duty factor with flight speed. It is thought that this modulation of duty factor permits birds to maintain relatively constant wing-beat kinematics resulting in maximum efficiency11, although it is still debated whether birds perform undulating flight because of kinematic and muscular constraints, rather than purely to save mechanical work10,11. It is therefore interesting that the elephant seal was able to satisfy mechanical power requirements for the same range of speeds using continuous and intermittent stroking, suggesting that intermittent locomotion primarily serves energy conservation in this species. However, the resultant saving is presumably a combination of efficiently converting muscular power into thrust, which relies on the interaction of an efficient muscle working stroke, coupled with limb kinematics that maximize the overall economy of converting fuel into forward locomotion.
Whereas the kinematics of individual undulations where relatively invariant in the pinnipeds, vertical movement during undulation in sharks displayed larger plasticity (Table 2). This variance was likely due to varying motivation of movement, given that large scale vertical movements serve both the purpose of travel and search in sharks23,24, which are characterized by different optima25. Indeed, the amplitude of individual undulations by sharks occurred over a wide vertical range (2–70 m, Fig. 1), whereas amplitudes were far more constrained in the two species of seal (Table 2). Despite the large variability in diving patterns of whale sharks, a recent study was able to show that continuous bounce diving utilized descent and ascent angles, which optimize the horizontal cost of transport25, supporting the notion that sharks also move through the water column in order to cover horizontal distance efficiently. These lines of evidence substantiate theoretical evidence of the efficacy of intermittent locomotion for the reduction of the cost of transport. Marine animals are often neutrally buoyant at depth, which yields insights into undulating flight as a gait in air and water. We found no evidence of intermittent locomotion by elephant seals at depths shallower than ∼15 m (Figs 2 and 3), probably as a result of animals being near neutral buoyancy (Fig. 2)26, whereas at deeper depths seals would swim intermittently, likely due to their increased overall density (Fig. 2). The lack of gas-spaces in sharks results in a fixed body density greater than that of water, irrespective of depth27,28. Thus, sharks can exhibit larger amplitudes in individual undulations than the two species of pinnipeds (Table 2). In fact, the amplitudes of the undulations in seals were so small that variations in body density are likely to be inconsequential within peaks and troughs of each undulation (Table 2 and Fig. 2), in a manner similar to the fixed negative buoyancy of sharks. However, there were significant differences in the depth at which the two elephant seals started to swim intermittently, likely resulting from differing body density (Fig. 2)29. Swimming gaits have been shown to relate to body condition in a number of species, such as baikal seals (Pusa sibirica) and weddel seals (Leptonychotes weddellii), with seals of higher body density adopting more gliding behaviour during descent30,31. It is, however, possible that a shift in behavioural mode, rather than buoyancy alone, triggered a change in the gait pattern of the elephant seals. For instance, some species of primate adopt intermittent locomotion to scan the environment, which may enable more efficient prey detection8. Against this, our elephant seal was on the outward leg of its migration moving rapidly over the Patagonian Shelf towards the shelf edge using u-shaped dives characteristic of directed travelling, whereas deep dives indicating foraging only occur once off-shelf waters are reached32 supporting the notion that energy conservation is the prime reason for intermittent locomotion.
Our results suggest that effective intermittent locomotion may require potential energy to be translated into horizontal distance and that seals have to exceed a critical body density to undulate effectively. Indeed, whereas duty factor increased systematically with depth in fur seals, which are characterized by appreciable air in their pelage, no such pattern was evident in whale sharks, presumably due to the lack of airspaces in sharks. Sharks, with their depth-independent negative buoyancy, appear to be able to glide continuously throughout the water column, whereas seals appear to be constrained to the depths were they attain negative buoyancy30. These results are at odds with 'burst and coast' swimming in small teleost fish, which may occur as a consequence of the profound difference in size between the fish observed thus far, and our study subjects. Indeed, it is possible that the cost of the burst acceleration phase is mass-specific33 and larger animals may benefit from less deceleration during the coast phase, which may be enhanced by negative buoyancy. In addition, locomotory activity during the active phase shows complete overlap with continuous locomotion, suggesting that undulating flight in large marine vertebrates is aerobic, unlike burst-coast swimming in smaller fish. The advantage of negative buoyancy as shown by our study has profound implications for our understanding of optimal body density in aquatic animals, where historically neutral buoyancy is often considered advantageous. In all likelihood, there is probably no single density that is optimal under all circumstances. Adjustment of density is likely highly adaptive, such that aquatic animals that exploit a prey patch at a given depth will benefit from neutral buoyancy34, while travelling animals may benefit from negative buoyancy (Figs. 2, 3). While many of the pelagic fish species have a reduced swim-bladder, there remains fine volume control over the size of the gas bladder.
Our findings indicate that intermittent locomotion in marine environments may be a common mode of transport that in all likelihood favours a lowering of aerobic costs due to efficient locomotion. By using animal-attached technologies to study submerged marine vertebrates, we demonstrate that this strategy operates in water across taxonomic clades as well as in air. Indeed, the species studied here all have to complete large-scale migrations between patches of productivity35,36,37, which would make efficient transiting particularly important. Any reduction in the cost of transport is expected to be highly selected for in natural populations and similar constraints are presumed to result in a convergence of morphological or behavioural solutions38. Given that animal movement is shaped by universal physical and physiological principles; such as commonalities regarding muscle efficiency39 and hydrodynamic interactions with the fluid environment40, common solutions might be expected to increase energetic efficiency even in differing media41. Indeed, sufficient lift-production during glides is the main criterion allowing birds to undulate11,42, whereas marine animals appear at times to face the opposite problem of not having enough 'effective weight' to undulate.
Methods
Field protocols
We instrumented four species of marine vertebrates from two distinct taxa with data-loggers containing acceleration and depth sensors to record changes in locomotory activity with depth. We attached multisensor data-loggers42, very high frequency (VHF) transmitters and platform terminal transmitters (PTTs) to the head of five lactating female southern elephant seals (M. leonina) at Peninsula Valdez (42° 44′ S 63° 38′ W), Argentina in October 2008. Animals were equipped before their migration following parturition32. Devices were recovered after animals returned from their ∼80 days foraging trip. Five data-loggers were deployed on lactating female northern fur seals (C. ursinus) on St Paul Island, Alaska (57°6′ N, 170°18′ W), USA during the breeding season of July 2008. Animals were captured and equipped in the rookery and devices recovered after a single foraging trip. Thirteen data-loggers were attached to whale sharks (R. typus) at Ningaloo Reef, Western Australia (22°00′S, 113°50′ E) between April and June from 2007–2009 (for full details of the equipment and procedure see Gleiss et al.4343). Three data-loggers were deployed on white sharks (C. carcharias) at the Farallon Islands (37°41′ N, 123°00′ W), central California in 2008 and 2009. Data-loggers were hidden in whale blubber obtained from stranding mortalities and freely ingested by sharks attracted to the research vessel. Acceleration data-loggers were attached to a satellite-linked Pop-up archival tag (MK10 Wildlife Computers) via wire-clamps (ClampTite). In addition, more syntactic foam was added to the package to offset the increased specific gravity by the acceleration data-logger. Loggers were recovered after the animal would regurgitate the package and it reported via pop-up satellite tag. By examining the location data provided by the ARGOS system the tag was recovered at sea using ultra high frequency antenna.
Device specifications
In this study, we employed two basic animal-attached recording systems: a multisensor data-logger42 and a pop-up satellite archival tag44. The multisensor data loggers used are described in detail in Wilson et al.42 In brief, acceleration and depth are recorded at a fixed frequency, which was determined according to the species in question (Table 1). Acceleration data were calibrated by rotating devices through known angles, so that device output corresponds to g (9.81 ms−2). Estimates of locomotory activity were derived through the proxy ODBA19, which scales linearly with oxygen consumption in exercising animals19,22, as a result of the interaction between muscular work and acceleration of the centre of mass21.
Calibration of the speed sensor
Only one of the elephant seals had a long enough data-record to adequately calibrate the on-board speed sensor, which has previously been described by Shepard et al.45. This sensor uses a reflective paddle and infrared sensor to measure in water speed, by measuring the amount of infrared reflectance from the paddle, which is a function of distance between the fixed sensor and flexible paddle. To calibrate device output to swimming speed46, we calculated the swimming speed for 10-s segments from animal pitch (via the surging acceleration) and vertical velocity (ascertained via pressure sensors) to gain an independent measure of travelling speed. This method is subject to increasingly large error as pitch nears horizontal and we therefore selected regions in the dataset where pitch was greater than 20° from the horizontal. We subsequently regressed calculated speeds with infrared device readings to obtain a predictive relationship, which we applied to the entire dataset. The instrument was only recording data for the portion of the migration where animals were swimming over the shelf, thus resulting in relatively shallow dives (<100 m) and correspondingly shallow pitch angles (mean pitch used to calculate speed was 45±13°), which resulted in larger variance in the calibration compared with other studies where only very steep pitch angles were used (velocity=1E+50*IR−12.44, n=51, r2=0.71)46.
Calculation of overall dynamic body acceleration
ODBA was calculated following the formulation presented in Wilson et al.19; static acceleration due to gravity was determined using a filter based on adjacent averaging. The size of the filtering window was determined using the method described in Shepard et al.47 Static acceleration was subsequently subtracted from the raw acceleration to yield dynamic acceleration (acceleration due to motion). This was performed for all three acceleration channels representing the three spatial planes. Absolute values were subsequently added to yield a single proxy for mechanical power output. Mathematically, this can be expresses as:

Where a acceleration in x, y or z axis, p is the smoothing window.
Data extraction
We defined undulating flight as a complete cycle in which the animal intersperses active propulsion with passive gliding, with depth increasing during glides and decreasing during active propulsion. We extracted individual undulations from all species when any flap-glide cycle began and ended at the same depth (we arbitrarily used ±15% offset between ascent and descent depth to qualify for this) in order to minimize error in calculating relative time spent stroking in relation to gliding. We then calculated the total cycle duration of each flap-glide cycle and the time spent gliding and actively stroking to calculate the duty factor the percentage of each movement cycle spent actively stroking10. Furthermore, we recorded minimum and maximum depth of each undulation to calculate the amplitude (change in depth over every flap-glide cycle) and the vertical velocity during the descent ('sink-rate') and the ascent ('climb-rate'). In addition, we extracted the mean ODBA for complete undulations as well as during continuous locomotion, in order to compare mean locomotory activity between the two gaits. For the elephant seal, we also manually extracted the mean swim speed for both continuous swimming and undulating flight. To compare the horizontal velocity of undulating flight with that of continuous locomotion, we adjusted the swim speed (V) to horizontal velocity (VHorizontal), using the absolute pitch angle of ascent and descent (Φ) calculated from the surging acceleration:

Additional information
How to cite this article: Gleiss, A.C. et al. Convergent evolution in locomotory patterns of flying and swimming animals. Nat. Commun. 2:352 doi: 10.1038/ncomms1351 (2011).
References
Schmidt-Nielsen, K. Locomotion: energy cost of swimming, flying, and running. Science 177, 222–228 (1972).
Alexander, R. M. & Bennet-Clark, H. C. Storage of elastic strain energy in muscle and other tissues. Nature 265, 114–117 (1977).
Feldkamp, S. Swimming in the California sea lion: morphometrics, drag and energetics. J. Exp. Biol. 131, 117–135 (1987).
Au, D. & Weihs, D. At high speeds dolphins save energy by leaping. Nature 284, 548–550 (1980).
Weihs, D. Tidal stream transport as an efficient method for migration. ICES J. Mar. Sci. 38, 92–99 (1978).
Herskin, J. & Steffensen, J. F. Energy savings in sea bass swimming in a school: measurements of tail beat frequency and oxygen consumption at different swimming speeds. J. Fish Biol. 53, 366–376 (1998).
Weimerskirch, H., Martin, J., Clerquin, Y., Alexandre, P. & Jiraskova, S. Energy saving in flight formation. Nature 413, 697–698 (2001).
Kramer, D. L. & McLaughlin, R. L. The behavioral ecology of intermittent locomotion. Am. Zool. 41, 137–153 (2001).
Weihs, D. Mechanically efficient swimming techniques for fish with negative buoyancy. J. Mar. Res. 31, 194–209 (1973).
Rayner, J. M. V., Viscardi, P. W., Ward, S. & Speakman, J. R. Aerodynamics and Energetics of Intermittent Flight in Birds. Am. Zool. 41, 188–204 (2001).
Tobalske, B. W. Morphology, velocity, and intermittent flight in birds. Am. Zool. 41, 177–187 (2001).
Tucker, V. A. Respiratory exchange and evaporative water loss in the flying budgerigar. J. Exp. Biol. 48, 67–87 (1968).
Williams, T. M. Intermittent swimming by mammals: a strategy for increasing energetic efficiency during diving. Am. Zool. 41, 166–176 (2001).
Williams, T. M. et al. Sink or swim: strategies for cost-efficient diving by marine mammals. Science 288, 133 (2000).
Skrovan, R., Williams, T., Berry, P., Moore, P. & Davis, R. The diving physiology of bottlenose dolphins (Tursiops truncatus). II. Biomechanics and changes in buoyancy at depth. J. Exp. Biol. 202, 2749–2761 (1999).
Davis, R. W. & Weihs, D. Locomotion in diving elephant seals: physical and physiological constraints. Philos. Trans. R. Soc. B Biol. Sci. 362, 2141 (2007).
Kawabe, R., Naito, Y., Sato, K., Miyashita, K. & Yamashita, N. Direct measurement of the swimming speed, tailbeat, and body angle of Japanese flounder (Paralichthys olivaceus). ICES J. Mar. Sci. 61, 1080–1087 (2004).
Rayner, J. M. V. Bounding and Undulating Flight in Birds. J. Theor. Biol. 117, 47–77 (1985).
Wilson, R. P. et al. Moving towards acceleration for estimates of activity-specific metabolic rate in free-living animals: the case of the cormorant. J. Anim. Ecol. 75, 1081–1090 (2006).
Fahlman, A., Wilson, R., Svärd, C., Rosen, D. A. S. & Trites, A. W. Activity and diving metabolism correlate in Steller sea lion Eumetopias jubatus. Aquat. Biol. 2, 75–84 (2008).
Gleiss, A. C., Wilson, R. P. & Shepard, E. L. C. Making overall dynamic body acceleration work: on the theory of acceleration as a proxy for energy expenditure. Methods Ecol. Evol. 2, 34–42 (2011).
Halsey, L. G. et al. The relationship between oxygen consumption and body acceleration in a range of species. Comp. Biochem. Physiol. Part A 152, 197–202 (2008).
Klimley, A. P., Beavers, S. C., Curtis, T. H. & Jorgensen, S. J. Movements and swimming behavior of three species of sharks in La Jolla Canyon, California. Environ. Biol. Fishes 63, 117–135 (2002).
Sims, D. W., Southall, E. J., Richardson, A. J., Reid, P. C. & Metcalfe, J. D. Seasonal movements and behaviour of basking sharks from archival tagging: no evidence of winter hibernation. Mar. Ecol. Prog. Ser. 248, 187–196 (2003).
Gleiss, A. C., Norman, B. & Wilson, R. P. Moved by that sinking feeling: variable diving geometry underlies movement strategies in whale sharks. Funct. Ecol. 25, 595–607 (2011).
Webb, P., Crocker, D., Blackwell, S., Costa, D. & Boeuf, B. Effects of buoyancy on the diving behavior of northern elephant seals. J. Exp. Biol. 201, 2349–2358 (1998).
Bone, Q. & Roberts, B. L. The density of elasmobranchs. J. Mar. Biol. Assoc. UK 49, 913–937 (1969).
Baldridge, H. D. Sinking factors and average densities of Florida sharks as functions of liver buoyancy. Copeia 4, 744–754 (1970).
Biuw, M., McConnell, B., Bradshaw, C. J. A., Burton, H. & Fedak, M. Blubber and buoyancy: monitoring the body condition of free-ranging seals using simple dive characteristics. J. Exp. Biol. 206, 3405–3423 (2003).
Watanabe, Y., Baranov, E. A., Sato, K., Naito, Y. & Miyazaki, N. Body density affects stroke patterns in Baikal seals. J. Exp. Biol. 209, 3269–3280 (2006).
Sato, K., Mitani, Y., Cameron, M. F., Siniff, D. B. & Naito, Y. Factors affecting stroking patterns and body angle in diving Weddell seals under natural conditions. J. Exp. Biol. 206, 1461–1470 (2003).
Campagna, C., Le Boeuf, B. J., Blackwell, S. B., Crocker, D. E. & Quintana, F. Diving behaviour and foraging location of female southern elephant seals from Patagonia. J. Zool. 236, 55–71 (1995).
Dial, K. P., Greene, E. & Irschick, D. J. Allometry of behavior. Trends Ecol. Evol. 23, 394–401 (2008).
Wilson, R. P. & Zimmer, I. Inspiration by Magellanic penguins: reduced swimming effort when under pressure. Mar. Ecol. Prog. Ser. 278, 303–307 (2004).
Lewis, M., Campagna, C. & Quintana, F. Site fidelity and dispersion of southern elephant selas from patagonia. Mar. Mamm. Sci. 12, 138–147 (1996).
Wilson, S. G., Polovina, J. J., Stewart, B. S. & Meekan, M. G. Movements of whale sharks (Rhincodon typus) tagged at Ningaloo Reef, Western Australia. Mar. Biol. 148, 1157–1166 (2006).
Jorgensen, S. J. et al. Philopatry and migration of Pacific white sharks. Proc. R. Soc. B Biol. Sci. 277, 679–688 (2010).
Morris, S. C. Evolutionary convergence. Curr. Biol. 16, R826–R827 (2006).
Hill, A. V. The effect of load on the heat of shortening of muscle. Proc. R. Soc. Lond. B Biol. Sci. (1934–1990) 159, 297–318 (1964).
Vogel, S. Life in Moving Fluids: The Physical Biology of Flow (Princeton Univ. Press, 1994).
Taylor, G. K., Nudds, R. L. & Thomas, A. L. R. Flying and swimming animals cruise at a Strouhal number tuned for high power efficiency. Nature 425, 707–711 (2003).
Wilson, R. P., Shepard, E. L. C. & Liebsch, N. Prying into the intimate details of animal lives: use of a daily diary on animals. Endanger. Species Res. 4, 123–137 (2008).
Gleiss, A. C., Norman, B., Liebsch, N., Francis, C. & Wilson, R. P. A new prospect for tagging large free-swimming sharks with motion-sensitive data-loggers. Fisheries Res. 97, 11–16 (2009).
Block, B. A., Dewar, H., Farwell, C. & Prince, E. D. A new satellite technology for tracking the movements of Atlantic bluefin tuna. Proc. Natl Acad. Sci. USA 95, 9384–9389 (1998).
Shepard, E. L. C. et al. Flexible paddle sheds new light on speed: a novel method for the remote measurement of swim speed in aquatic animals. Endanger. Species Res. 4, 157–164 (2008).
Goldbogen, J. A. et al. Kinematics of foraging dives and lunge-feeding in fin whales. J. Exp. Biol. 209, 1231–1244 (2006).
Shepard, E. L. C et al. Derivation of body motion via appropriate smoothing of acceleration data. Aquat. Biol. 4, 235–241 (2009).
Acknowledgements
A.C.G. was funded by a Wingate Foundation Scholarship and a Swansea University Research Fees Scholarship. Fieldwork on Elephant Seals and Whale Sharks was funded by grants from the National Geographic Society (EC0407-08, ECO423-09), as well as a Rolex Award for Enterprise. Whale Shark fieldwork was funded by Royal Caribbean Cruise Lines (Ocean Fund) and Rolex Award for Enterprise under permit by the Western Australian Department of Environment and Conservation. The northern fur seal research was supported by the North Pacific Research Board under permit no. 715-1884 issued by the US National Marine Fisheries Service. Additional support was provided by NOAA and the North Pacific Marine Science Foundation through the North Pacific Universities Marine Mammal Research Consortium. White Shark Fieldwork was funded by the Sloan, Moore and Packard Foundations as a part of the Tagging of Pacic Pelagics programme of the Census of Marine Life. The Monterey Bay Aquarium Foundation also provided nancial support. S. Anderson, T. Chapple, the crew of RV Bayliss and RV Bo Kooling, L. Longley, G. Shedrawi, H. Shortland-Jones, D. Morgan, S. Lindfield, D. Bradley and E.Wilson provided field support, Randy Davis and Sabrina Fossette provided valuable comments to an earlier version of the manuscript.
Author information
Authors and Affiliations
Contributions
A.C.G., R.P.W., G.C.H. and S.J.J. conceived the study. A.C.G., S.J.J., N.L., J.E.S., B.N., F.Q. and R.P.W. performed the fieldwork. E.G. created the animations. A.C.G. and S.J.J. analysed the data. A.C.G. drafted the paper, with contributions from S.J.J., N.L., J.E.S., B.N., G.C.H., F.Q., E.G., C.C., A.W.T., B.A.B. and R.P.W.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Movie 1
An animated whale shark moving through the water column using undulating flight. All movements and orientation are directly drawn from the in situ collected data from acceleration and depth sensors. Static acceleration was used animate create body orientation and dynamic swaying acceleration was employed to animate movement of the caudal fin. (AVI 3491 kb)
Rights and permissions
About this article
Cite this article
Gleiss, A., Jorgensen, S., Liebsch, N. et al. Convergent evolution in locomotory patterns of flying and swimming animals. Nat Commun 2, 352 (2011). https://doi.org/10.1038/ncomms1350
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms1350
This article is cited by
-
Vortex interactions of two burst-and-coast swimmers in a side-by-side arrangement
Theoretical and Computational Fluid Dynamics (2023)
-
Large size in aquatic tetrapods compensates for high drag caused by extreme body proportions
Communications Biology (2022)
-
What’s in a resource gradient? Comparing alternative cues for foraging in dynamic environments via movement, perception, and memory
Theoretical Ecology (2022)
-
Accelerometry to study fine-scale activity of invasive Burmese pythons (Python bivittatus) in the wild
Animal Biotelemetry (2021)
-
Hidden Markov models identify major movement modes in accelerometer and magnetometer data from four albatross species
Movement Ecology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.