Abstract
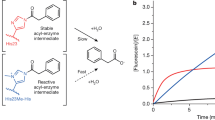
Despite their unparalleled catalytic prowess and environmental compatibility, enzymes have yet to see widespread application in synthetic chemistry. This lack of application and the resulting underuse of their enormous potential stems not only from a wariness about aqueous biological catalysis on the part of the typical synthetic chemist but also from limitations on enzyme applicability that arise from the high degree of substrate specificity possessed by most enzymes. This latter perceived limitation is being successfully challenged through rational protein engineering1,2 and directed evolution efforts3,4,5,6 to alter substrate specificity. However, such programs require considerable effort to establish. Here we report an alternative strategy for expanding the substrate specificity, and therefore the synthetic utility, of a given enzyme through a process of 'substrate engineering'. The attachment of a readily removable functional group to an alternative glycosyltransferase substrate induces a productive binding mode, facilitating rational control of substrate specificity and regioselectivity using wild-type enzymes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
References
Craik, C.S. et al. Redesigning trypsin—alteration of substrate-specificity. Science 228, 291–297 (1985).
Wells, J.A., Powers, D.B., Bott, R.R., Graycar, T.P. & Estell, D.A. Designing substrate specificity by protein engineering of electrostatic interactions. Proc. Natl. Acad. Sci. USA 84, 1219–1223 (1987).
Ghadessy, F.J., Ong, J.L. & Holliger, P. Directed evolution of polymerase function by compartmentalized self-replication. Proc. Natl. Acad. Sci. USA 98, 4552–4557 (2001).
Griffiths, A.D. & Tawfik, D.S. Directed evolution of an extremely fast phosphotriesterase by in vitro compartmentalization. EMBO J. 22, 24–35 (2003).
Tao, H.Y. & Cornish, V.W. Milestones in directed enzyme evolution. Curr. Opin. Chem. Biol. 6, 858–864 (2002).
Bloom, J.D. et al. Evolving strategies for enzyme engineering. Curr. Opin. Struct. Biol. 15, 447–452 (2005).
Davies, G., Sinnott, M.L. & Withers, S.G. Glycosyl transfer. in Comprehensive Biological Catalysis (ed. Sinnott, M.L.) 119–208 (Academic Press, San Diego, 1998).
Coutinho, P.M., Deleury, E., Davies, G.J. & Henrissat, B. An evolving hierarchical family classification for glycosyltransferases. J. Mol. Biol. 328, 307–317 (2003).
Lairson, L.L. & Withers, S.G. Mechanistic analogies amongst carbohydrate modifying enzymes. Chem. Commun. 2243–2248 (2004).
Wakarchuk, W.W. et al. Dependence of the bi-functional nature of a sialyltransferase from Neisseria meningitidis on a single amino acid substitution. J. Biol. Chem. 276, 12785–12790 (2001).
Rich, J.R., Szpacenko, A., Palcic, M.M. & Bundle, D.R. Glycosyltransferase-catalyzed synthesis of thiooligosaccharides. Angew. Chem. Int. Ed. 43, 613–615 (2004).
Bencur, P. et al. Arabidopsis thaliana β-1,2-xylosyltransferase: an unusual glycosyltransferase with the potential to act at multiple stages of the plant N-glycosylation pathway. Biochem. J. 388, 515–525 (2005).
Durr, C. et al. The glycosyltransferase UrdGT2 catalyzes both C- and O-glycosidic sugar transfers. Angew. Chem. Int. Ed. 43, 2962–2965 (2004).
Yang, M. et al. Probing the breadth of macrolide glycosyltransferases: in vitro remodeling of a polyketide antibiotic creates active bacterial uptake and enhances potency. J. Am. Chem. Soc. 127, 9336–9337 (2005).
Borisova, S.A., Zhao, L., Melancon, C.E., Kao, C.L. & Liu, H.W. Characterization of the glycosyltransferase activity of DesVII: analysis of and implications for the biosynthesis of macrolide antibiotics. J. Am. Chem. Soc. 126, 6534–6535 (2004).
Oberthur, M. et al. A systematic investigation of the synthetic utility of glycopeptide glycosyltransferases. J. Am. Chem. Soc. 127, 10747–10752 (2005).
Blanco, G. et al. Identification of a sugar flexible glycosyltransferase from Streptomyces olivaceus, the producer of the antitumor polyketide elloramycin. Chem. Biol. 8, 253–263 (2001).
Ly, H.D., Lougheed, B., Wakarchuk, W.W. & Withers, S.G. Mechanistic studies of a retaining α-galactosyltransferase from Neisseria meningitidis. Biochemistry 41, 5075–5085 (2002).
Persson, K. et al. Crystal structure of the retaining galactosyltransferase LgtC from Neisseria meningitidis in complex with donor and acceptor sugar analogs. Nat. Struct. Biol. 8, 166–175 (2001).
Lougheed, B., Ly, H.D., Wakarchuk, W.W. & Withers, S.G. Glycosyl fluorides can function as substrates for nucleotide phosphosugar-dependent glycosyltransferases. J. Biol. Chem. 274, 37717–37722 (1999).
Nerinckx, W., Desmet, T. & Claeyssens, M. A hydrophobic platform as a mechanistically relevant transition state stabilising factor appears to be present in the active centre of all glycoside hydrolases. FEBS Lett. 538, 1–7 (2003).
Chiu, C.P.C. et al. Structural analysis of the sialyltransferase CstII from Campylobacter jejuni in complex with a substrate analog. Nat. Struct. Mol. Biol. 11, 163–170 (2004).
Gastinel, L.N. et al. Bovine α1,3-galactosyltransferase catalytic domain structure and its relationship with ABO histo-blood group and glycosphingolipid glycosyltransferases. EMBO J. 20, 638–649 (2001).
Zhang, Y. et al. Roles of active site tryptophans in substrate binding and catalysis by α-1,3 galactosyltransferase. Glycobiology 14, 1295–1302 (2004).
Mackenzie, L.F., Wang, Q.P., Warren, R.A.J. & Withers, S.G. Glycosynthases: mutant glycosidases for oligosaccharide synthesis. J. Am. Chem. Soc. 120, 5583–5584 (1998).
Nashiru, O. β-Mannosynthase: synthesis of β-mannosides with a mutant β-mannosidase. Angew. Chem. Int. Ed. 40, 417–420 (2001).
Miura, Y., Arai, T. & Yamagata, T. Synthesis of amphiphilic lactosides that possess a lactosylceramide-mimicking N-acyl structure: alternative universal substrates for endo-type glycosylceramidases. Carbohydr. Res. 289, 193–199 (1996).
Stick, R.V., Stubbs, K.A. & Watts, A.G. Modifying the regioselectivity of glycosynthase reactions through changes in the acceptor. Aust. J. Chem. 57, 779–786 (2004).
Carter, P. & Wells, J.A. Engineering enzyme specificity by “substrate-assisted catalysis”. Science 237, 394–399 (1987).
Murakami, H., Ohta, A., Ashigai, H. & Suga, H. A highly flexible tRNA acylation method for non-natural polypeptide synthesis. Nat. Methods 3, 357–359 (2006).
Acknowledgements
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canadian Institutes for Health Research (CIHR) and the Protein Engineering Network of Centres of Excellence of Canada (PENCE). L.L.L is the recipient of a Michael Smith Foundation for Health Research (MSFHR) Senior Graduate Studentship and a NSERC doctoral postgraduate scholarship.
Author information
Authors and Affiliations
Contributions
L.L. and A.W. conceived of this project, which was refined with input from S.W. and W.W. L.L. performed essentially all of the experimental work and, in conjunction with S.W., wrote the manuscript with input from A.W. and W.W.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
1H-COSY NMR spectra of 19. (PDF 205 kb)
Supplementary Fig. 2
Alternative CSt II acceptor substrates. (PDF 92 kb)
Supplementary Fig. 3
1H-COSY NMR spectra of 22. (PDF 197 kb)
Supplementary Fig. 4
1H-COSY NMR spectra of 23. (PDF 207 kb)
Supplementary Fig. 5
1H-COSY NMR spectra of 24. (PDF 182 kb)
Supplementary Fig. 6
1H-COSY NMR spectra of 28. (PDF 98 kb)
Supplementary Fig. 7
1H-COSY NMR spectra of 29. (PDF 137 kb)
Rights and permissions
About this article
Cite this article
Lairson, L., Watts, A., Wakarchuk, W. et al. Using substrate engineering to harness enzymatic promiscuity and expand biological catalysis. Nat Chem Biol 2, 724–728 (2006). https://doi.org/10.1038/nchembio828
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio828
This article is cited by
-
Perspectives for biocatalytic lignin utilization: cleaving 4-O-5 and Cα–Cβ bonds in dimeric lignin model compounds catalyzed by a promiscuous activity of tyrosinase
Biotechnology for Biofuels (2017)
-
Towards tailor-made oligosaccharides—chemo-enzymatic approaches by enzyme and substrate engineering
Applied Microbiology and Biotechnology (2009)
-
A sweet success for substrate engineering
Nature Chemical Biology (2006)