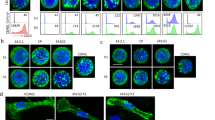
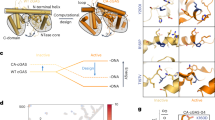
Abstract
Interaction between CD40, a member of the tumor necrosis factor receptor (TNFR) superfamily, and its ligand CD40L, a 39-kDa glycoprotein, is essential for the development of humoral and cellular immune responses1,2. Selective blockade or activation of this pathway provides the ground for the development of new treatments against immunologically based diseases3,4 and malignancies5,6. Like other members of the TNF superfamily, CD40L monomers self-assemble around a threefold symmetry axis to form noncovalent homotrimers that can each bind three receptor molecules7,8. Here, we report on the structure-based design of small synthetic molecules with C3 symmetry that can mimic CD40L homotrimers. These molecules interact with CD40, compete with the binding of CD40L to CD40, and reproduce, to a certain extent, the functional properties of the much larger homotrimeric soluble CD40L. Architectures based on rigid C3-symmetric cores may thus represent a general approach to mimicking homotrimers of the TNF superfamily.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
van Kooten, C. & Banchereau, J. CD40–CD40 ligand. J. Leukoc. Biol. 67, 1–17 (2000).
Diehl, L. et al. The role of CD40 in peripheral T cell tolerance and immunity. J. Mol. Med. 78, 363–371 (2000).
Howard, L.M. et al. Mechanisms of immunotherapeutic intervention by anti-CD40L (CD154) antibody in an animal model of multiple sclerosis. J. Clin. Invest. 103, 281–290 (1999).
Kirk, A.D. et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat. Med. 5, 686–693 (1999).
Diehl, L. et al. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat. Med. 5, 774–779 (1999).
Sotomayor, E.M. et al. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nat. Med. 5, 780–787 (1999).
Bodmer, J.-L., Schneider, P. & Tschopp, J. The molecular architecture of the TNF superfamily. Trends Biochem. Sci. 27, 19–26 (2002).
Karpusas, M. et al. 2 Å crystal structure of an extracellular fragment of human CD40 ligand. Structure 3, 1031–1039 (1995).
Schoenberger, S.P., Toes, R.E., van der Voort, E.I., Offringa, R. & Melief, C.J. T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature 393, 480–483 (1998).
Bennett, S.R. et al. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 393, 478–480 (1998).
Cella, M. et al. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 184, 747–752 (1996).
Chaussabel, D. et al. CD40 ligation prevents Trypanosoma cruzi infection through interleukin-12 upregulation. Infect. Immun. 67, 1929–1934 (1999).
Edelmann, K.H. & Wilson, C.B. Role of CD28/CD80–86 and CD40/CD154 costimulatory interactions in host defense to primary herpes simplex virus infection. J. Virol. 75, 612–621 (2001).
Heldin, C.-H. Dimerization of cell surface receptors in signal transduction. Cell 80, 213–223 (1995).
Liu, Y. et al. Ligand-receptor binding revealed by the TNF family member TALL-1. Nature 423, 49–56 (2003).
Hymowitz, S.G. et al. Structures of APRIL-receptor complexes: like BCMA, TACI employs only a single cysteine-rich domain for high affinity ligand binding. J. Biol. Chem. 280, 7218–7227 (2005).
Wrighton, N.C. et al. Small peptides as potent mimetics of the protein hormone erythropoietin. Science 273, 458–464 (1996).
Cwirla, S.E. et al. Peptide agonist of the thrombopoietin receptor as potent as the natural cytokine. Science 276, 1696–1699 (1997).
Whitty, A. & Borysenko, C.W. Small molecule cytokine mimetics. Chem. Biol. 6, R107–R118 (1999).
Mammen, M., Choi, S.-K. & Whitesides, G.M. Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew. Chem. Int. Edn Engl. 37, 2754–2794 (1998).
Kiessling, L.L., Gestwicki, J.E. & Strong, L.E. Synthetic multivalent ligands in the exploration of cell-surface interactions. Curr. Opin. Chem. Biol. 4, 696–703 (2000).
Singh, J. et al. The role of polar interactions in the molecular recognition of CD40L with its receptor CD40. Protein Sci. 7, 1124–1135 (1998).
Bajorath, J. et al. Analysis of gp39/CD40 interactions using molecular models and site-directed mutagenesis. Biochemistry 34, 9884–9892 (1995).
Tong, A.W. et al. CD40 ligand-induced apoptosis is Fas-independent in human multiple myeloma cells. Leuk. Lymphoma 36, 543–558 (2000).
Pound, J.D. et al. Minimal cross-linking and epitope requirements for CD40-dependent suppression of apoptosis contrast with those for promotion of the cell cycle and homotypic adhesions in human B cells. Int. Immunol. 11, 11–20 (1999).
Schneider, P. et al. Conversion of membrane-bound Fas (CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J. Exp. Med. 187, 1205–1213 (1998).
Haswell, L.E., Glennie, M.J. & Al-Shamkhani, A. Analysis of the oligomeric requirement for signaling by CD40 using soluble multimeric forms of its ligand, CD154. Eur. J. Immunol. 31, 3094–3100 (2001).
Schuurhuis, D.H. et al. Immature dendritic cells acquire CD8(+) cytotoxic T lymphocyte priming capacity upon activation by T helper cell-independent or -dependent stimuli. J. Exp. Med. 192, 145–150 (2000).
O'Sullivan, B.J. & Thomas, R. CD40 ligation conditions dendritic cell antigen-presenting function through sustained activation of NF-kappaB. J. Immunol. 168, 5491–5498 (2002).
Lortat-Jacob, H., Chouin, E., Cusack, S. & van Raaij, M.J. Kinetic analysis of adenovirus fiber binding to its receptor reveals an avidity mechanism for trimeric receptor-ligand interactions. J. Biol. Chem. 276, 9009–9015 (2001).
Acknowledgements
We thank J.-P. Briand and S. Muller for support; J. Mutterer for assistance with confocal microscopy; N. Bonnefoy-Berard and M. Flacher for providing Burkitt lymphoma cells and M. Monestier for providing LG11-2 mAb. This work was supported by the Centre National de la Recherche Scientifique, the Ministère de la Recherche (ACI Jeunes Chercheurs), La Ligue contre le Cancer, Région Alsace and the Agence Nationale de Recherches contre le SIDA. S.W. was supported by a grant from the Ministère de la Recherche. W.S. was supported by the Ministère de la Recherche and the Fondation pour la Recherche Médicale. N.T. was supported by La Ligue Contre le Cancer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Binding of anti-human CD40 mAb 5C3 to CD40 does not prevent subsequent binding of 1 or hCD40L:CD8 as seen by SPR experiments. (PDF 149 kb)
Supplementary Fig. 2
Scanning single amino acid substitutions within the CD40 binding motif of 1. (PDF 84 kb)
Supplementary Fig. 3
Compound 11, a biotinylated analog of ligand 2 binds to CD40, induces lymphoma apoptosis and interacts with membrane-associated CD40 molecule. (PDF 46 kb)
Supplementary Fig. 4
Maturation of the murine dendritic cell line D1 by CD40L mimetics. (PDF 138 kb)
Supplementary Fig. 5
Preparation and characterization of CD40L mimetic 1. (PDF 117 kb)
Supplementary Table 1
Summary of inhibition experiments and measured kinetic values for the interaction of the different ligands with CD40. (PDF 83 kb)
Rights and permissions
About this article
Cite this article
Fournel, S., Wieckowski, S., Sun, W. et al. C3-symmetric peptide scaffolds are functional mimetics of trimeric CD40L. Nat Chem Biol 1, 377–382 (2005). https://doi.org/10.1038/nchembio746
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio746
This article is cited by
-
TNF receptor 2 pathway: drug target for autoimmune diseases
Nature Reviews Drug Discovery (2010)
-
Small-molecule costimulatory blockade: organic dye inhibitors of the CD40–CD154 interaction
Journal of Molecular Medicine (2009)
-
Vaccine leads to memory loss
Nature Medicine (2007)
-
TNFR superfamily trimers
Nature Reviews Drug Discovery (2006)
-
Toward small-molecule agonists of TNF receptors
Nature Chemical Biology (2005)