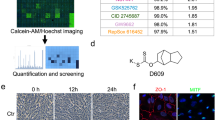
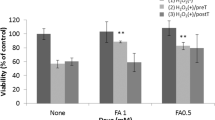
Abstract
Vertebrate vision is initiated by photoisomerization of the visual pigment chromophore 11-cis-retinal and is maintained by continuous regeneration of this retinoid through a series of reactions termed the retinoid cycle. However, toxic side reaction products, especially those involving reactive aldehyde groups of the photoisomerized product, all-trans-retinal, can cause severe retinal pathology. Here we lowered peak concentrations of free all-trans-retinal with primary amine–containing Food and Drug Administration (FDA)–approved drugs that did not inhibit chromophore regeneration in mouse models of retinal degeneration. Schiff base adducts between all-trans-retinal and these amines were identified by MS. Adducts were observed in mouse eyes only when an experimental drug protected the retina from degeneration in both short-term and long-term treatment experiments. This study demonstrates a molecular basis of all-trans-retinal–induced retinal pathology and identifies an assemblage of FDA-approved compounds with protective effects against this pathology in a mouse model that shows features of Stargardt's disease and age-related retinal degeneration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Palczewski, K. G protein–coupled receptor rhodopsin. Annu. Rev. Biochem. 75, 743–767 (2006).
Palczewski, K. et al. Crystal structure of rhodopsin: a G protein–coupled receptor. Science 289, 739–745 (2000).
Travis, G.H., Golczak, M., Moise, A.R. & Palczewski, K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu. Rev. Pharmacol. Toxicol. 47, 469–512 (2007).
von Lintig, J., Kiser, P.D., Golczak, M. & Palczewski, K. The biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision. Trends Biochem. Sci. 35, 400–410 (2010).
Rattner, A., Smallwood, P.M. & Nathans, J. Identification and characterization of all-trans-retinol dehydrogenase from photoreceptor outer segments, the visual cycle enzyme that reduces all-trans-retinal to all-trans-retinol. J. Biol. Chem. 275, 11034–11043 (2000).
Molday, R.S., Beharry, S., Ahn, J. & Zhong, M. Binding of N-retinylidene-PE to ABCA4 and a model for its transport across membranes. Adv. Exp. Med. Biol. 572, 465–470 (2006).
Maeda, A., Maeda, T., Golczak, M. & Palczewski, K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J. Biol. Chem. 283, 26684–26693 (2008).
Golczak, M., Kuksa, V., Maeda, T., Moise, A.R. & Palczewski, K. Positively charged retinoids are potent and selective inhibitors of the trans-cis isomerization in the retinoid (visual) cycle. Proc. Natl. Acad. Sci. USA 102, 8162–8167 (2005).
Maeda, A. et al. Effects of potent inhibitors of the retinoid cycle on visual function and photoreceptor protection from light damage in mice. Mol. Pharmacol. 70, 1220–1229 (2006).
Golczak, M. et al. Lecithin:retinol acyltransferase is responsible for amidation of retinylamine, a potent inhibitor of the retinoid cycle. J. Biol. Chem. 280, 42263–42273 (2005).
Maeda, A. et al. Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J. Biol. Chem. 284, 15173–15183 (2009).
Allikmets, R. et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat. Genet. 15, 236–246 (1997).
Cremers, F.P. et al. Autosomal recessive retinitis pigmentosa and cone-rod dystrophy caused by splice site mutations in the Stargardt's disease gene ABCR. Hum. Mol. Genet. 7, 355–362 (1998).
Martínez-Mir, A. et al. Retinitis pigmentosa caused by a homozygous mutation in the Stargardt disease gene ABCR. Nat. Genet. 18, 11–12 (1998).
Zhang, Q. et al. Severe autosomal recessive retinitis pigmentosa maps to chromosome 1p13.3-p21.2 between D1S2896 and D1S457 but outside ABCA4. Hum. Genet. 118, 356–365 (2005).
Allikmets, R. Further evidence for an association of ABCR alleles with age-related macular degeneration. The International ABCR Screening Consortium. Am. J. Hum. Genet. 67, 487–491 (2000).
Tsybovsky, Y., Molday, R.S. & Palczewski, K. The ATP-binding cassette transporter ABCA4: structural and functional properties and role in retinal disease. Adv. Exp. Med. Biol. 703, 105–125 (2010).
Molday, R.S. & Zhang, K. Defective lipid transport and biosynthesis in recessive and dominant Stargardt macular degeneration. Prog. Lipid. Res. 49, 476–492 (2010).
Zhong, M., Molday, L.L. & Molday, R.S. Role of the C terminus of the photoreceptor ABCA4 transporter in protein folding, function, and retinal degenerative diseases. J. Biol. Chem. 284, 3640–3649 (2009).
Maeda, A. et al. Redundant and unique roles of retinol dehydrogenases in the mouse retina. Proc. Natl. Acad. Sci. USA 104, 19565–19570 (2007).
Sparrow, J.R., Wu, Y., Kim, C.Y. & Zhou, J. Phospholipid meets all-trans-retinal: the making of RPE bisretinoids. J. Lipid Res. 51, 247–261 (2010).
Parish, C.A., Hashimoto, M., Nakanishi, K., Dillon, J. & Sparrow, J. Isolation and one-step preparation of A2E and iso-A2E, fluorophores from human retinal pigment epithelium. Proc. Natl. Acad. Sci. USA 95, 14609–14613 (1998).
Murdaugh, L.S. et al. Compositional studies of human RPE lipofuscin. J. Mass Spectrom. 45, 1139–1147 (2010).
Różanowska, M. & Sarna, T. Light-induced damage to the retina: role of rhodopsin chromophore revisited. Photochem. Photobiol. 81, 1305–1330 (2005).
Negre-Salvayre, A., Coatrieux, C., Ingueneau, C. & Salvayre, R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br. J. Pharmacol. 153, 6–20 (2008).
Ellis, E.M. Reactive carbonyls and oxidative stress: potential for therapeutic intervention. Pharmacol. Ther. 115, 13–24 (2007).
Sun, H. & Nathans, J. ABCR, the ATP-binding cassette transporter responsible for Stargardt macular dystrophy, is an efficient target of all-trans-retinal–mediated photooxidative damage in vitro. Implications for retinal disease. J. Biol. Chem. 276, 11766–11774 (2001).
Mata, N.L., Weng, J. & Travis, G.H. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc. Natl. Acad. Sci. USA 97, 7154–7159 (2000).
Kim, Y.K. et al. Retinyl ester formation by lecithin: retinol acyltransferase is a key regulator of retinoid homeostasis in mouse embryogenesis. J. Biol. Chem. 283, 5611–5621 (2008).
Fishkin, N., Yefidoff, R., Gollipalli, D.R. & Rando, R.R. On the mechanism of isomerization of all-trans-retinol esters to 11-cis-retinol in retinal pigment epithelial cells: 11-fluoro-all-trans-retinol as substrate/inhibitor in the visual cycle. Bioorg. Med. Chem. 13, 5189–5194 (2005).
Yannuzzi, L.A. et al. Ophthalmic fundus imaging: today and beyond. Am. J. Ophthalmol. 137, 511–524 (2004).
Nickell, S., Park, P.S., Baumeister, W. & Palczewski, K. Three-dimensional architecture of murine rod outer segments determined by cryoelectron tomography. J. Cell Biol. 177, 917–925 (2007).
Holz, F.G. et al. Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am. J. Ophthalmol. 143, 463–472 (2007).
Imanishi, Y., Batten, M.L., Piston, D.W., Baehr, W. & Palczewski, K. Noninvasive two-photon imaging reveals retinyl ester storage structures in the eye. J. Cell Biol. 164, 373–383 (2004).
Taylor, C.P. et al. Potent and stereospecific anticonvulsant activity of 3-isobutyl GABA relates to in vitro binding at a novel site labeled by tritiated gabapentin. Epilepsy Res. 14, 11–15 (1993).
Field, M.J., Oles, R.J. & Singh, L. Pregabalin may represent a novel class of anxiolytic agents with a broad spectrum of activity. Br. J. Pharmacol. 132, 1–4 (2001).
Golczak, M. et al. Metabolic basis of visual cycle inhibition by retinoid and nonretinoid compounds in the vertebrate retina. J. Biol. Chem. 283, 9543–9554 (2008).
Dean, D.M., Nguitragool, W., Miri, A., McCabe, S.L. & Zimmerman, A.L. All-trans-retinal shuts down rod cyclic nucleotide-gated ion channels: a novel role for photoreceptor retinoids in the response to bright light? Proc. Natl. Acad. Sci. USA 99, 8372–8377 (2002).
Maeda, A. et al. Palmitoylation stabilizes unliganded rod opsin. Proc. Natl. Acad. Sci. USA 107, 8428–8433 (2010).
Maeda, A. et al. Retinol dehydrogenase (RDH12) protects photoreceptors from light-induced degeneration in mice. J. Biol. Chem. 281, 37697–37704 (2006).
Kalia, J. & Raines, R.T. Hydrolytic stability of hydrazones and oximes. Angew. Chem. Int. Edn Engl. 47, 7523–7526 (2008).
Weng, J. et al. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt's disease from the phenotype in abcr knockout mice. Cell 98, 13–23 (1999).
Mata, N.L. et al. Delayed dark-adaptation and lipofuscin accumulation in abcr+/− mice: implications for involvement of ABCR in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 42, 1685–1690 (2001).
Sparrow, J.R., Nakanishi, K. & Parish, C.A. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Invest. Ophthalmol. Vis. Sci. 41, 1981–1989 (2000).
Vollmer-Snarr, H.R. et al. Amino-retinoid compounds in the human retinal pigment epithelium. Adv. Exp. Med. Biol. 572, 69–74 (2006).
Maeda, A. et al. Role of photoreceptor-specific retinol dehydrogenase in the retinoid cycle in vivo. J. Biol. Chem. 280, 18822–18832 (2005).
Kim, S.R. et al. Rpe65 Leu450Met variant is associated with reduced levels of the retinal pigment epithelium lipofuscin fluorophores A2E and iso-A2E. Proc. Natl. Acad. Sci. USA 101, 11668–11672 (2004).
Stecher, H. & Palczewski, K. Multienzyme analysis of visual cycle. Methods Enzymol. 316, 330–344 (2000).
Stecher, H., Gelb, M.H., Saari, J.C. & Palczewski, K. Preferential release of 11-cis-retinol from retinal pigment epithelial cells in the presence of cellular retinaldehyde-binding protein. J. Biol. Chem. 274, 8577–8585 (1999).
Acknowledgements
We would like to thank Z. Dong for expert handling of mice, S. Roos for block preparation and plastic sectioning, J. Zhang for NMR analyses, and M. Matosky for retinoid analyses. We also thank L. Webster Jr, J. Saari, M.E. Maguire and members of the Palczewski laboratory for critical comments on the manuscript. This research was supported in part by grants EY009339, EY021126, EY019031, EY019880 and P30 EY11373 from the US National Institutes of Health; TECH 09–004 from the State of Ohio Department of Development; the Third Frontier Commission; Research to Prevent Blindness; Ohio Lion Eye Research Foundation; Foundation Fighting Blindness; and Fight for Sight.
Author information
Authors and Affiliations
Contributions
A.M., M.G. and K.P. conceived and directed the project. A.M., M.G., T.M., Y.C., K.O., H.K., S.S. and K.I. designed and conducted experiments. G.P. and K.P. performed pharmacological analyses of the data. A.M., M.G. and K.P. prepared the manuscript. A.M., M.G., G.P., W.H. and T.M. analyzed the data and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
Case Western Reserve University and Visum Inc. may commercialize some of the technology described in this work. A.M., M.G. and T.M. are consultants for Visum Inc., and K.P. and W.H. are cofounders of Visum Inc.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Results (PDF 2260 kb)
Rights and permissions
About this article
Cite this article
Maeda, A., Golczak, M., Chen, Y. et al. Primary amines protect against retinal degeneration in mouse models of retinopathies. Nat Chem Biol 8, 170–178 (2012). https://doi.org/10.1038/nchembio.759
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.759
This article is cited by
-
Clinical application of ultra-widefield fundus autofluorescence
International Ophthalmology (2021)
-
Microfluidics-based green synthesis of silver nanoparticle from the aqueous leaf extract of Ipomea quamoclit L.
Applied Nanoscience (2021)
-
Preclinical pharmacology of a lipophenol in a mouse model of light-induced retinopathy
Experimental & Molecular Medicine (2020)
-
A novel small molecule chaperone of rod opsin and its potential therapy for retinal degeneration
Nature Communications (2018)
-
Clinical applications of fundus autofluorescence in retinal disease
International Journal of Retina and Vitreous (2016)