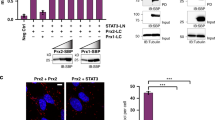
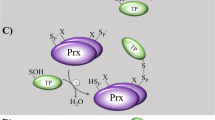
Abstract
Hydrogen peroxide (H2O2) acts as a signaling messenger by triggering the reversible oxidation of redox-regulated proteins. It remains unclear how proteins can be oxidized by signaling levels of H2O2 in the presence of peroxiredoxins, which are highly efficient peroxide scavengers. Here we show that the rapid formation of disulfide bonds in cytosolic proteins is enabled, rather than competed, by cytosolic 2-Cys peroxiredoxins. Under the conditions tested, the combined deletion or depletion of cytosolic peroxiredoxins broadly frustrated H2O2-dependent protein thiol oxidation, which is the exact opposite of what would be predicted based on the assumption that H2O2 oxidizes proteins directly. We find that peroxiredoxins enable rapid and sensitive protein thiol oxidation by relaying H2O2-derived oxidizing equivalents to other proteins. Although these findings do not rule out the existence of Prx-independent H2O2 signaling mechanisms, they suggest a broader role for peroxiredoxins as sensors and transmitters of H2O2 signals than hitherto recognized.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Holmström, K.M. & Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 15, 411–421 (2014).
Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 11, 613–619 (2017).
Stone, J.R. & Yang, S. Hydrogen peroxide: a signaling messenger. Antioxid. Redox Signal. 8, 243–270 (2006).
García-Santamarina, S., Boronat, S. & Hidalgo, E. Reversible cysteine oxidation in hydrogen peroxide sensing and signal transduction. Biochemistry 53, 2560–2580 (2014).
Stone, J.R. An assessment of proposed mechanisms for sensing hydrogen peroxide in mammalian systems. Arch. Biochem. Biophys. 422, 119–124 (2004).
Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 4, 278–286 (2008).
Brigelius-Flohé, R. & Flohé, L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid. Redox Signal. 15, 2335–2381 (2011).
Marinho, H.S., Real, C., Cyrne, L., Soares, H. & Antunes, F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2, 535–562 (2014).
Winterbourn, C.C. & Hampton, M.B. Thiol chemistry and specificity in redox signaling. Free Radic. Biol. Med. 45, 549–561 (2008).
Winterbourn, C.C. & Peskin, A.V. Kinetic approaches to measuring peroxiredoxin reactivity. Mol. Cells 39, 26–30 (2016).
Chae, H.Z., Kim, H.J., Kang, S.W. & Rhee, S.G. Characterization of three isoforms of mammalian peroxiredoxin that reduce peroxides in the presence of thioredoxin. Diabetes Res. Clin. Pract. 45, 101–112 (1999).
Randall, L.M., Ferrer-Sueta, G. & Denicola, A. Peroxiredoxins as preferential targets in H2O2-induced signaling. Methods Enzymol. 527, 41–63 (2013).
Winterbourn, C.C. & Hampton, M.B. Redox biology: signaling via a peroxiredoxin sensor. Nat. Chem. Biol. 11, 5–6 (2015).
Delaunay, A., Pflieger, D., Barrault, M.B., Vinh, J. & Toledano, M.B. A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell 111, 471–481 (2002).
Veal, E.A. et al. A 2-Cys peroxiredoxin regulates peroxide-induced oxidation and activation of a stress-activated MAP kinase. Mol. Cell 15, 129–139 (2004).
Calvo, I.A. et al. Dissection of a redox relay: H2O2-dependent activation of the transcription factor Pap1 through the peroxidatic Tpx1-thioredoxin cycle. Cell Rep. 5, 1413–1424 (2013).
Jarvis, R.M., Hughes, S.M. & Ledgerwood, E.C. Peroxiredoxin 1 functions as a signal peroxidase to receive, transduce, and transmit peroxide signals in mammalian cells. Free Radic. Biol. Med. 53, 1522–1530 (2012).
Sobotta, M.C. et al. Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nat. Chem. Biol. 11, 64–70 (2015).
Sobotta, M.C. et al. Exposing cells to H2O2: a quantitative comparison between continuous low-dose and one-time high-dose treatments. Free Radic. Biol. Med. 60, 325–335 (2013).
Schwertassek, U. et al. Reactivation of oxidized PTP1B and PTEN by thioredoxin 1. FEBS J. 281, 3545–3558 (2014).
Madureira, P.A. & Waisman, D.M. Annexin A2: the importance of being redox sensitive. Int. J. Mol. Sci. 14, 3568–3594 (2013).
Nadeau, P.J., Charette, S.J., Toledano, M.B. & Landry, J. Disulfide bond-mediated multimerization of Ask1 and its reduction by thioredoxin-1 regulate H2O2-induced c-Jun NH2-terminal kinase activation and apoptosis. Mol. Biol. Cell 18, 3903–3913 (2007).
Ferrer-Sueta, G. et al. Factors affecting protein thiol reactivity and specificity in peroxide reduction. Chem. Res. Toxicol. 24, 434–450 (2011).
Flohé, L. The impact of thiol peroxidases on redox regulation. Free Radic. Res. 50, 126–142 (2016).
Forman, H.J., Maiorino, M. & Ursini, F. Signaling functions of reactive oxygen species. Biochemistry 49, 835–842 (2010).
Netto, L.E. & Antunes, F. The roles of peroxiredoxin and thioredoxin in hydrogen peroxide sensing and in signal transduction. Mol. Cells 39, 65–71 (2016).
Perkins, A., Nelson, K.J., Parsonage, D., Poole, L.B. & Karplus, P.A. Peroxiredoxins: guardians against oxidative stress and modulators of peroxide signaling. Trends Biochem. Sci. 40, 435–445 (2015).
Schwertassek, U., Weingarten, L. & Dick, T.P. Identification of redox-active cell-surface proteins by mechanism-based kinetic trapping. Sci. STKE 2007, pl8 (2007).
Nordberg, J. & Arnér, E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 31, 1287–1312 (2001).
Debarbieux, L. & Beckwith, J. On the functional interchangeability, oxidant versus reductant, of members of the thioredoxin superfamily. J. Bacteriol. 182, 723–727 (2000).
García-Santamarina, S. et al. Is oxidized thioredoxin a major trigger for cysteine oxidation? Clues from a redox proteomics approach. Antioxid. Redox Signal. 18, 1549–1556 (2013).
Li, L., Cheung, S.H., Evans, E.L. & Shaw, P.E. Modulation of gene expression and tumor cell growth by redox modification of STAT3. Cancer Res. 70, 8222–8232 (2010).
Morinaka, A. et al. Thioredoxin mediates oxidation-dependent phosphorylation of CRMP2 and growth cone collapse. Sci. Signal. 4, ra26 (2011).
Peralta, D. et al. A proton relay enhances H2O2 sensitivity of GAPDH to facilitate metabolic adaptation. Nat. Chem. Biol. 11, 156–163 (2015).
Truong, T.H. et al. Molecular basis for redox activation of epidermal growth factor receptor kinase. Cell Chem. Biol. 23, 837–848 (2016).
Antunes, F. & Brito, P.M. Quantitative biology of hydrogen peroxide signaling. Redox Biol. 13, 1–7 (2017).
Travasso, R.D.M., Sampaio Dos Aidos, F., Bayani, A., Abranches, P. & Salvador, A. Localized redox relays as a privileged mode of cytoplasmic hydrogen peroxide signaling. Redox Biol. 12, 233–245 (2017).
Anestål, K., Prast-Nielsen, S., Cenas, N. & Arnér, E.S. Cell death by SecTRAPs: thioredoxin reductase as a prooxidant killer of cells. PLoS One 3, e1846 (2008).
Stöcker, S., Van Laer, K., Mijuskovic, A. & Dick, T.P. The conundrum of hydrogen peroxide signaling and the emerging role of peroxiredoxins as redox relay hubs. Antioxid. Redox Signal. http://dx.doi.org/10.1089/ars.2017.7162 (2017).
Fomenko, D.E. et al. Thiol peroxidases mediate specific genome-wide regulation of gene expression in response to hydrogen peroxide. Proc. Natl. Acad. Sci. USA 108, 2729–2734 (2011).
Morgan, B. et al. Real-time monitoring of basal H2O2 levels with peroxiredoxin-based probes. Nat. Chem. Biol. 12, 437–443 (2016).
Rhee, S.G. & Kil, I.S. Multiple functions and regulation of mammalian peroxiredoxins. Annu. Rev. Biochem. 86, 749–775 (2017).
Park, M.H., Jo, M., Kim, Y.R., Lee, C.K. & Hong, J.T. Roles of peroxiredoxins in cancer, neurodegenerative diseases and inflammatory diseases. Pharmacol. Ther. 163, 1–23 (2016).
Perkins, A., Poole, L.B. & Karplus, P.A. Tuning of peroxiredoxin catalysis for various physiological roles. Biochemistry 53, 7693–7705 (2014).
Schwertassek, U. et al. Selective redox regulation of cytokine receptor signaling by extracellular thioredoxin-1. EMBO J. 26, 3086–3097 (2007).
Wagner, B.A., Witmer, J.R., van 't Erve, T.J. & Buettner, G.R. An assay for the rate of removal of extracellular hydrogen peroxide by cells. Redox Biol. 1, 210–217 (2013).
Kempe, H., Schwabe, A., Crémazy, F., Verschure, P.J. & Bruggeman, F.J. The volumes and transcript counts of single cells reveal concentration homeostasis and capture biological noise. Mol. Biol. Cell 26, 797–804 (2015).
Park, J.W., Piszczek, G., Rhee, S.G. & Chock, P.B. Glutathionylation of peroxiredoxin I induces decamer to dimers dissociation with concomitant loss of chaperone activity. Biochemistry 50, 3204–3210 (2011).
Acknowledgements
We thank G. Kuntz for general technical assistance. We thank S. Merker and R. Hardt for technical assistance with mass spectrometry. We thank B. Jovanovic and G. Stöcklin for technical advice. We acknowledge support by the Deutsche Forschungsgemeinschaft (SFB1036 to T.P.D. and T.R.) and by the European Research Council (742039 to T.P.D.).
Author information
Authors and Affiliations
Contributions
T.P.D. and S.S. designed the project and wrote the paper. S.S. performed the experiments. M.M. contributed to Figure 4. T.R. analyzed mass spectrometry data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Table 1, Supplementary Figures 1–12 (PDF 22104 kb)
Rights and permissions
About this article
Cite this article
Stöcker, S., Maurer, M., Ruppert, T. et al. A role for 2-Cys peroxiredoxins in facilitating cytosolic protein thiol oxidation. Nat Chem Biol 14, 148–155 (2018). https://doi.org/10.1038/nchembio.2536
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2536