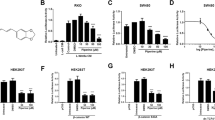
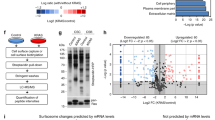
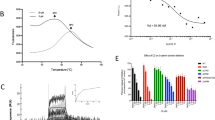
Abstract
Wnt (wingless)/β-catenin signaling is critical for tumor progression and is frequently activated in colorectal cancer as a result of the mutation of adenomatous polyposis coli (APC); however, therapeutic agents targeting this pathway for clinical use are lacking. Here we report that nitazoxanide (NTZ), a clinically approved antiparasitic drug, efficiently inhibits Wnt signaling independent of APC. Using chemoproteomic approaches, we have identified peptidyl arginine deiminase 2 (PAD2) as the functional target of NTZ in Wnt inhibition. By targeting PAD2, NTZ increased the deamination (citrullination) and turnover of β-catenin in colon cancer cells. Replacement of arginine residues disrupted the transcriptional activity, and NTZ induced degradation of β-catenin. In Wnt-activated colon cancer cells, knockout of either PAD2 or β-catenin substantially increased resistance to NTZ treatment. Our data highlight the potential of NTZ as a modulator of β-catenin citrullination for the treatment of cancer patients with Wnt pathway mutations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Li, V.S. et al. Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell 149, 1245–1256 (2012).
Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, 330–337 (2012).
Ding, L. et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature 455, 1069–1075 (2008).
Grasso, C.S. et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 487, 239–243 (2012).
Jia, D. et al. Exome sequencing of hepatoblastoma reveals novel mutations and cancer genes in the Wnt pathway and ubiquitin ligase complex. Hepatology 60, 1686–1696 (2014).
Jones, D.T. et al. Dissecting the genomic complexity underlying medulloblastoma. Nature 488, 100–105 (2012).
Kandoth, C. et al. Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature 497, 67–73 (2013).
Reya, T. & Clevers, H. Wnt signalling in stem cells and cancer. Nature 434, 843–850 (2005).
Harada, N. et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 18, 5931–5942 (1999).
Kahn, M. Can we safely target the WNT pathway? Nat. Rev. Drug Discov. 13, 513–532 (2014).
Kandoth, C. et al. Mutational landscape and significance across 12 major cancer types. Nature 502, 333–339 (2013).
Sato, N., Meijer, L., Skaltsounis, L., Greengard, P. & Brivanlou, A.H. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat. Med. 10, 55–63 (2004).
Fuerer, C. & Nusse, R. Lentiviral vectors to probe and manipulate the Wnt signaling pathway. PLoS One 5, e9370 (2010).
Heckl, D. et al. Generation of mouse models of myeloid malignancy with combinatorial genetic lesions using CRISPR-Cas9 genome editing. Nat. Biotechnol. 32, 941–946 (2014).
Chan, T.A., Wang, Z., Dang, L.H., Vogelstein, B. & Kinzler, K.W. Targeted inactivation of CTNNB1 reveals unexpected effects of beta-catenin mutation. Proc. Natl. Acad. Sci. USA 99, 8265–8270 (2002).
Hudson, C., Kawai, N., Negishi, T. & Yasuo, H. β-Catenin-driven binary fate specification segregates germ layers in ascidian embryos. Curr. Biol. 23, 491–495 (2013).
Schneider, S.Q. & Bowerman, B. β-Catenin asymmetries after all animal/vegetal- oriented cell divisions in Platynereis dumerilii embryos mediate binary cell-fate specification. Dev. Cell 13, 73–86 (2007).
Watanabe, K. et al. Integrative ChIP-seq/microarray analysis identifies a CTNNB1 target signature enriched in intestinal stem cells and colon cancer. PLoS One 9, e92317 (2014).
Su, L.K. et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 256, 668–670 (1992).
Theodos, C.M., Griffiths, J.K., D'Onfro, J., Fairfield, A. & Tzipori, S. Efficacy of nitazoxanide against Cryptosporidium parvum in cell culture and in animal models. Antimicrob. Agents Chemother. 42, 1959–1965 (1998).
Bajaj, J., Zimdahl, B. & Reya, T. Fearful symmetry: subversion of asymmetric division in cancer development and progression. Cancer Res. 75, 792–797 (2015).
Su, Y. et al. APC is essential for targeting phosphorylated β-catenin to the SCFbeta-TrCP ubiquitin ligase. Mol. Cell 32, 652–661 (2008).
Lomenick, B. et al. Target identification using drug affinity responsive target stability (DARTS). Proc. Natl. Acad. Sci. USA 106, 21984–21989 (2009).
Wienken, C.J., Baaske, P., Rothbauer, U., Braun, D. & Duhr, S. Protein-binding assays in biological liquids using microscale thermophoresis. Nat. Commun. 1, 100 (2010).
Martinez Molina, D. et al. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science 341, 84–87 (2013).
Fuhrmann, J., Clancy, K.W. & Thompson, P.R. Chemical biology of protein arginine modifications in epigenetic regulation. Chem. Rev. 115, 5413–5461 (2015).
Lewallen, D.M. et al. Chemical proteomic platform to identify citrullinated proteins. ACS Chem. Biol. 10, 2520–2528 (2015).
Slade, D.J. et al. Protein arginine deiminase 2 binds calcium in an ordered fashion: implications for inhibitor design. ACS Chem. Biol. 10, 1043–1053 (2015).
Bang, H. et al. Mutation and citrullination modifies vimentin to a novel autoantigen for rheumatoid arthritis. Arthritis Rheum. 56, 2503–2511 (2007).
Ashiru, O., Howe, J.D. & Butters, T.D. Nitazoxanide, an antiviral thiazolide, depletes ATP-sensitive intracellular Ca2+ stores. Virology 462–463, 135–148 (2014).
Huels, D.J. et al. E-cadherin can limit the transforming properties of activating β-catenin mutations. EMBO J. 34, 2321–2333 (2015).
Anderson, V.R. & Curran, M.P. Nitazoxanide: a review of its use in the treatment of gastrointestinal infections. Drugs 67, 1947–1967 (2007).
Di Santo, N. & Ehrisman, J. A functional perspective of nitazoxanide as a potential anticancer drug. Mutat. Res. 768, 16–21 (2014).
Hsu, K.W. et al. The activated Notch1 receptor cooperates with alpha-enolase and MBP-1 in modulating c-myc activity. Mol. Cell. Biol. 28, 4829–4842 (2008).
Senkowski, W. et al. Three-dimensional cell culture-based screening identifies the anthelmintic drug nitazoxanide as a candidate for treatment of colorectal cancer. Mol. Cancer Ther. 14, 1504–1516 (2015).
Rossignol, J.F., Elfert, A., El-Gohary, Y. & Keeffe, E.B. Improved virologic response in chronic hepatitis C genotype 4 treated with nitazoxanide, peginterferon, and ribavirin. Gastroenterology 136, 856–862 (2009).
Yue, X. et al. Hepatitis B virus-induced calreticulin protein is involved in IFN resistance. J. Immunol. 189, 279–286 (2012).
Korba, B.E., Elazar, M., Lui, P., Rossignol, J.F. & Glenn, J.S. Potential for hepatitis C virus resistance to nitazoxanide or tizoxanide. Antimicrob. Agents Chemother. 52, 4069–4071 (2008).
Ramírez, G., Valck, C., Ferreira, V.P., López, N. & Ferreira, A. Extracellular Trypanosoma cruzi calreticulin in the host-parasite interplay. Trends Parasitol. 27, 115–122 (2011).
Müller, J. et al. Thiazolides inhibit growth and induce glutathione-S-transferase Pi (GSTP1)-dependent cell death in human colon cancer cells. Int. J. Cancer 123, 1797–1806 (2008).
Fan-Minogue, H. et al. A c-Myc activation sensor-based high-throughput drug screening identifies an antineoplastic effect of nitazoxanide. Mol. Cancer Ther. 12, 1896–1905 (2013).
Brockmann, A. et al. Structure-function relationship of thiazolide-induced apoptosis in colorectal tumor cells. ACS Chem. Biol. 9, 1520–1527 (2014).
Stadler, S.C. et al. Dysregulation of PAD4-mediated citrullination of nuclear GSK3β activates TGF-β signaling and induces epithelial-to-mesenchymal transition in breast cancer cells. Proc. Natl. Acad. Sci. USA 110, 11851–11856 (2013).
Deplus, R. et al. Citrullination of DNMT3A by PADI4 regulates its stability and controls DNA methylation. Nucleic Acids Res. 42, 8285–8296 (2014).
Vossenaar, E.R., Zendman, A.J., van Venrooij, W.J. & Pruijn, G.J. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. BioEssays 25, 1106–1118 (2003).
Wu, H.Y. et al. Structural basis of antizyme-mediated regulation of polyamine homeostasis. Proc. Natl. Acad. Sci. USA 112, 11229–11234 (2015).
Van Lidth de Jeude, J.F., Vermeulen, J.L., Montenegro-Miranda, P.S., Van den Brink, G.R. & Heijmans, J. A protocol for lentiviral transduction and downstream analysis of intestinal organoids. J. Vis. Exp. 2015, e52531 (2015).
Qu, Y. et al. Generation of prostate tumor-initiating cells is associated with elevation of reactive oxygen species and IL-6/STAT3 signaling. Cancer Res. 73, 7090–7100 (2013).
Acknowledgements
We thank H.M. Hoang for DNA microarray profiling, M. Eidsheim for immunohistochemistry and S.M. Leh for clinical materials. We thank C. Lv for the calcium measurement, and X. Zu and X. Liu for the LC–Q-TOF–MS analysis. We thank the Flow Cytometry Core Facility, Department of Clinical Science, University of Bergen. We thank R. Nusse (Stanford University, Stanford, CA) for the Wnt reporter 7TGC, O.J. Sansom (Cancer Research UK Beatson Institute, Glasgow, UK) for the mutant Apc and Ctnnb1 organoids, and S. Coonrod for the PAD2 expression vector and valuable discussion. We acknowledge funding from Einar Galtung Døsvig, Espen Galtung Døsvig, Jan Einar Greve, Bjarne Rieber, Herman Friele, Trond Mohn, Thorstein Selvik, Kåre Rommetveit, Tordis and Fritz C. Rieber's legacy to K.H.K., Bergen Research Foundation to X.K., Helse Vest grants 911778 to Y.Q., 911626 and 912062 to K.H.K., 911747 to X.K., Professor of Chang Jiang Scholars Program and NSFC (81230090, 81520108030) to W.Z., the Society for Skin Cancer Research to M.P.L. and the EU Horizon 2020 Collaborative Research Project SOUND (633974) to P.F.C.
Author information
Authors and Affiliations
Contributions
Y.Q. and X.K. designed the project, performed experiments, analyzed data, interpreted results and wrote the manuscript; J.R.O. performed experiments; X.Y. and W.Z. performed mice experiments, LC–Q-TOF–MS analysis and calcium measurement; P. F. C. and M.P.L. performed data analysis; P.S.H. provided materials; A.M.O. performed DNA microarray profiling; K.A.B. performed immunohistochemistry staining; K.-H.K. designed the project, interpreted results and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
X.S., Y.Q., K.H.K. & A.M.O. are listed as inventors of the patent-pending (PCT/EP2016/076171) filed by Bergen Teknologioverføring AS.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Tables 1–3 and Supplementary Figures 1–23 (PDF 4628 kb)
Rights and permissions
About this article
Cite this article
Qu, Y., Olsen, J., Yuan, X. et al. Small molecule promotes β-catenin citrullination and inhibits Wnt signaling in cancer. Nat Chem Biol 14, 94–101 (2018). https://doi.org/10.1038/nchembio.2510
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2510
This article is cited by
-
Role of non-canonical post-translational modifications in gastrointestinal tumors
Cancer Cell International (2023)
-
Target identification of small molecules: an overview of the current applications in drug discovery
BMC Biotechnology (2023)
-
Macrophage’s role in solid tumors: two edges of a sword
Cancer Cell International (2023)
-
Nitazoxanide inhibits acetylated KLF5-induced bone metastasis by modulating KLF5 function in prostate cancer
BMC Medicine (2023)
-
Peptidylarginine deiminase 2 plays a key role in osteogenesis by enhancing RUNX2 stability through citrullination
Cell Death & Disease (2023)