Abstract
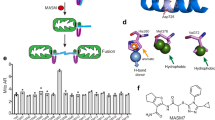
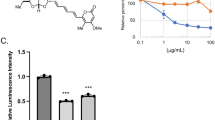
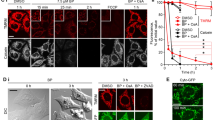
Tim17 and Tim23 are the main subunits of the TIM23 complex, one of the two major essential mitochondrial inner-membrane protein translocon machineries (TIMs). No chemical probes that specifically inhibit TIM23-dependent protein import were known to exist. Here we show that the natural product stendomycin, produced by Streptomyces hygroscopicus, is a potent and specific inhibitor of the TIM23 complex in yeast and mammalian cells. Furthermore, stendomycin-mediated blockage of the TIM23 complex does not alter normal processing of the major regulatory mitophagy kinase PINK1, but TIM23 is required to stabilize PINK1 on the outside of mitochondria to initiate mitophagy upon membrane depolarization.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Aoun, M. & Tiranti, V. Mitochondria: a crossroads for lipid metabolism defect in neurodegeneration with brain iron accumulation diseases. Int. J. Biochem. Cell Biol. 63, 25–31 (2015).
Bhola, P.D. & Letai, A. Mitochondria-judges and executioners of cell death Sentences. Mol. Cell 61, 695–704 (2016).
Endo, T., Yamamoto, H. & Esaki, M. Functional cooperation and separation of translocators in protein import into mitochondria, the double-membrane bounded organelles. J. Cell Sci. 116, 3259–3267 (2003).
Chacinska, A., Koehler, C.M., Milenkovic, D., Lithgow, T. & Pfanner, N. Importing mitochondrial proteins: machineries and mechanisms. Cell 138, 628–644 (2009).
MacKenzie, J.A. & Payne, R.M. Mitochondrial protein import and human health and disease. Biochim. Biophys. Acta. 1772, 509–523 (2007).
Pfanner, N. & Meijer, M. Mitochondrial biogenesis: the Tom and Tim machine. Curr. Biol. 7, R100–R103 (1997).
Thompson, R.Q. & Hughes, M.S. Stendomycin: A new antifungal antibiotic. J. Antibiot. (Tokyo) 16, 187–194 (1963).
Hoepfner, D. et al. High-resolution chemical dissection of a model eukaryote reveals targets, pathways and gene functions. Microbiol. Res. 169, 107–120 (2014).
Saleem, A., Iqbal, S., Zhang, Y. & Hood, D.A. Effect of p53 on mitochondrial morphology, import, and assembly in skeletal muscle. Am. J. Physiol. Cell Physiol. 308, C319–C329 (2015).
Mokranjac, D. & Neupert, W. The many faces of the mitochondrial TIM23 complex. Biochim. Biophys. Acta. 1797, 1045–1054 (2010).
Sirrenberg, C., Bauer, M.F., Guiard, B., Neupert, W. & Brunner, M. Import of carrier proteins into the mitochondrial inner membrane mediated by Tim22. Nature 384, 582–585 (1996).
Jensen, R.E. & Johnson, A.E. Protein translocation: is Hsp70 pulling my chain? Curr. Biol. 9, R779–R782 (1999).
Demishtein-Zohary, K. et al. Role of Tim17 in coupling the import motor to the translocation channel of the mitochondrial presequence translocase. eLife 6, e22696 (2017).
Chacinska, A. et al. Mitochondrial presequence translocase: switching between TOM tethering and motor recruitment involves Tim21 and Tim17. Cell 120, 817–829 (2005).
Greene, A.W. et al. Mitochondrial processing peptidase regulates PINK1 processing, import and Parkin recruitment. EMBO Rep. 13, 378–385 (2012).
Rüb, C., Wilkening, A. & Voos, W. Mitochondrial quality control by the Pink1/Parkin system. Cell Tissue Res. 367, 111–123 (2017).
Becker, D., Richter, J., Tocilescu, M.A., Przedborski, S. & Voos, W. Pink1 kinase and its membrane potential (ΔΨ)-dependent cleavage product both localize to outer mitochondrial membrane by unique targeting mode. J. Biol. Chem. 287, 22969–22987 (2012).
Lazarou, M., Jin, S.M., Kane, L.A. & Youle, R.J. Role of PINK1 binding to the TOM complex and alternate intracellular membranes in recruitment and activation of the E3 ligase Parkin. Dev. Cell 22, 320–333 (2012).
Hasson, S.A. et al. High-content genome-wide RNAi screens identify regulators of parkin upstream of mitophagy. Nature 504, 291–295 (2013).
Kersten, R.D. et al. A mass spectrometry-guided genome mining approach for natural product peptidogenomics. Nat. Chem. Biol. 7, 794–802 (2011).
Ungermann, C., Guiard, B., Neupert, W. & Cyr, D.M. The delta psi- and Hsp70/MIM44-dependent reaction cycle driving early steps of protein import into mitochondria. EMBO J. 15, 735–744 (1996).
Schneider, H.-C. et al. Mitochondrial Hsp70/MIM44 complex facilitates protein import. Nature 371, 768–774 (1994).
Simorre, J.P., Genest, D., Caille, A. & Ptak, M. A 2D NMR study of the internal flexibility of the antifungal peptide stendomycin. Eur. Biophys. J. 18, 309–316 (1990).
Huang, Z. et al. A functional variomics tool for discovering drug-resistance genes and drug targets. Cell Rep. 3, 577–585 (2013).
Pries, V. et al. Advantages and challenges of phenotypic screens: the identification of two novel antifungal geranylgeranyltransferase I inhibitors. J. Biomol. Screen. 21, 306–315 (2016).
Glick, B.S. & Pon, L.A. Isolation of highly purified mitochondria from Saccharomyces cerevisiae. Methods Enzymol. 260, 213–223 (1995).
Claypool, S.M., Whited, K., Srijumnong, S., Han, X. & Koehler, C.M. Barth syndrome mutations that cause tafazzin complex lability. J. Cell Biol. 192, 447–462 (2011).
Acknowledgements
The authors acknowledge the NIH GM61721 to C.M.K. and DFG STE 2045/1-1 to J.S. The authors also wish to thank the rest of the Novartis HIP HOP team.
Author information
Authors and Affiliations
Contributions
I.F. and J.S. designed and performed experiments, analyzed the data and wrote the paper; M.G., L.G., M.A.C., C.P., R.C. and M.P., designed and performed experiments and analyzed the data; P.K. and D.H. supervised experiments and analyzed the data; J.B. designed experiments and analyzed the data; C.M.K. and S.B.H. designed experiments, analyzed the data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
I.F., M.G., L.G., C.P., R.C., M.P., P.K., D.H., J.B. and S.B.H. are current or former employees of Novartis and may hold stock in the company.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Figures 1–5 and Supplementary Note. (PDF 22313 kb)
Rights and permissions
About this article
Cite this article
Filipuzzi, I., Steffen, J., Germain, M. et al. Stendomycin selectively inhibits TIM23-dependent mitochondrial protein import. Nat Chem Biol 13, 1239–1244 (2017). https://doi.org/10.1038/nchembio.2493
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2493
This article is cited by
-
Molecular pathway of mitochondrial preprotein import through the TOM–TIM23 supercomplex
Nature Structural & Molecular Biology (2023)
-
The cellular response to drug perturbation is limited: comparison of large-scale chemogenomic fitness signatures
BMC Genomics (2022)
-
N-degron-mediated degradation and regulation of mitochondrial PINK1 kinase
Current Genetics (2020)
-
PINK1 import regulation; a fine system to convey mitochondrial stress to the cytosol
BMC Biology (2018)