Abstract
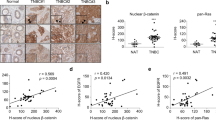
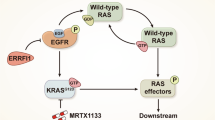
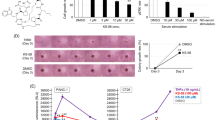
Both the Wnt/β-catenin and Ras pathways are aberrantly activated in most human colorectal cancers (CRCs) and interact cooperatively in tumor promotion. Inhibition of these signaling may therefore be an ideal strategy for treating CRC. We identified KY1220, a compound that destabilizes both β-catenin and Ras, via targeting the Wnt/β-catenin pathway, and synthesized its derivative KYA1797K. KYA1797K bound directly to the regulators of G-protein signaling domain of axin, initiating β-catenin and Ras degradation through enhancement of the β-catenin destruction complex activating GSK3β. KYA1797K effectively suppressed the growth of CRCs harboring APC and KRAS mutations, as shown by various in vitro studies and by in vivo studies using xenograft and transgenic mouse models of tumors induced by APC and KRAS mutations. Destabilization of both β-catenin and Ras via targeting axin is a potential therapeutic strategy for treatment of CRC and other type cancers activated Wnt/β-catenin and Ras pathways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hawk, E.T. & Levin, B. Colorectal cancer prevention. J. Clin. Oncol. 23, 378–391 (2005).
Jemal, A. et al. Cancer statistics, 2009. CA Cancer J. Clin. 59, 225–249 (2009).
Waldner, M.J. & Neurath, M.F. The molecular therapy of colorectal cancer. Mol. Aspects Med. 31, 171–178 (2010).
Chung, D.C. The genetic basis of colorectal cancer: insights into critical pathways of tumorigenesis. Gastroenterology 119, 854–865 (2000).
Zhang, B. et al. β-Catenin and ras oncogenes detect most human colorectal cancer. Clin. Cancer Res. 9, 3073–3079 (2003).
Kinzler, K.W. & Vogelstein, B. Lessons from hereditary colorectal cancer. Cell 87, 159–170 (1996).
Bos, J.L. et al. Prevalence of ras gene mutations in human colorectal cancers. Nature 327, 293–297 (1987).
Brink, M. et al. K-ras oncogene mutations in sporadic colorectal cancer in The Netherlands Cohort Study. Carcinogenesis 24, 703–710 (2003).
Janssen, K.P. et al. APC and oncogenic KRAS are synergistic in enhancing Wnt signaling in intestinal tumor formation and progression. Gastroenterology 131, 1096–1109 (2006).
D'Abaco, G.M., Whitehead, R.H. & Burgess, A.W. Synergy between Apc min and an activated ras mutation is sufficient to induce colon carcinomas. Mol. Cell. Biol. 16, 884–891 (1996).
Luo, F. et al. Mutated K-ras(Asp12) promotes tumourigenesis in Apc(Min) mice more in the large than the small intestines, with synergistic effects between K-ras and Wnt pathways. Int. J. Exp. Pathol. 90, 558–574 (2009).
Fearon, E.R. & Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 61, 759–767 (1990).
Sansom, O.J. et al. Loss of Apc allows phenotypic manifestation of the transforming properties of an endogenous K-ras oncogene in vivo. Proc. Natl. Acad. Sci. USA 103, 14122–14127 (2006).
Anastas, J.N. & Moon, R.T. WNT signalling pathways as therapeutic targets in cancer. Nat. Rev. Cancer 13, 11–26 (2013).
Guardavaccaro, D. & Clevers, H. Wnt/beta-catenin and MAPK signaling: allies and enemies in different battlefields. Sci. Signal. 5, pe15 (2012).
Jeong, W.J. et al. Ras stabilization through aberrant activation of Wnt/beta-catenin signaling promotes intestinal tumorigenesis. Sci. Signal. 5, ra30 (2012).
Jeon, S.H. et al. Axin inhibits extracellular signal-regulated kinase pathway by Ras degradation via beta-catenin. J. Biol. Chem. 282, 14482–14492 (2007).
Park, K.S. et al. APC inhibits ERK pathway activation and cellular proliferation induced by RAS. J. Cell Sci. 119, 819–827 (2006).
Kim, S.E. et al. H-Ras is degraded by Wnt/beta-catenin signaling via beta-TrCP-mediated polyubiquitylation. J. Cell Sci. 122, 842–848 (2009).
Moon, B.S. et al. Role of oncogenic K-Ras in cancer stem cell activation by aberrant Wnt/beta-catenin signaling. J. Natl. Cancer Inst. 106, djt373 (2014).
Yun, J. et al. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science 325, 1555–1559 (2009).
Arnold, H.K. et al. The Axin1 scaffold protein promotes formation of a degradation complex for c-Myc. EMBO J. 28, 500–512 (2009).
Yost, C. et al. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 10, 1443–1454 (1996).
Chen, B. et al. Small molecule–mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 5, 100–107 (2009).
Huang, S.M. et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461, 614–620 (2009).
Taketo, M.M. & Edelmann, W. Mouse models of colon cancer. Gastroenterology 136, 780–798 (2009).
Johnson, L. et al. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature 410, 1111–1116 (2001).
Loeb, K.R. & Loeb, L.A. Significance of multiple mutations in cancer. Carcinogenesis 21, 379–385 (2000).
Loeb, L.A., Loeb, K.R. & Anderson, J.P. Multiple mutations and cancer. Proc. Natl. Acad. Sci. USA 100, 776–781 (2003).
Tortora, G. et al. Overcoming resistance to molecularly targeted anticancer therapies: Rational drug combinations based on EGFR and MAPK inhibition for solid tumours and haematologic malignancies. Drug Resist. Updat. 10, 81–100 (2007).
Fearon, E.R. Molecular genetics of colorectal cancer. Annu. Rev. Pathol. 6, 479–507 (2011).
Kikuchi, A. Roles of axin in the Wnt signalling pathway. Cell. Signal. 11, 777–788 (1999).
Yun, M.S., Kim, S.E., Jeon, S.H., Lee, J.S. & Choi, K.Y. Both ERK and Wnt/beta-catenin pathways are involved in Wnt3a-induced proliferation. J. Cell Sci. 118, 313–322 (2005).
Hoeflich, K.P. et al. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature 406, 86–90 (2000).
Mo, H. & Elson, C.E. Apoptosis and cell-cycle arrest in human and murine tumor cells are initiated by isoprenoids. J. Nutr. 129, 804–813 (1999).
Kim, M.Y., Kaduwal, S., Yang, D.H. & Choi, K.Y. Bone morphogenetic protein 4 stimulates attachment of neurospheres and astrogenesis of neural stem cells in neurospheres via phosphatidylinositol 3 kinase-mediated upregulation of N-cadherin. Neuroscience 170, 8–15 (2010).
Ha, N.C., Tonozuka, T., Stamos, J.L., Choi, H.J. & Weis, W.I. Mechanism of phosphorylation-dependent binding of APC to beta-catenin and its role in beta-catenin degradation. Mol. Cell 15, 511–521 (2004).
Kim, S. & Jho, E.H. The protein stability of Axin, a negative regulator of Wnt signaling, is regulated by Smad ubiquitination regulatory factor 2 (Smurf2). J. Biol. Chem. 285, 36420–36426 (2010).
Kolligs, F.T., Hu, G., Dang, C.V. & Fearon, E.R. Neoplastic transformation of RK3E by mutant beta-catenin requires deregulation of Tcf/Lef transcription but not activation of c-myc expression. Mol. Cell. Biol. 19, 5696–5706 (1999).
Winawer, S. et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology 124, 544–560 (2003).
Acknowledgements
We thank B. Vogelstein, K.W. Kinzler, J.-W. Oh, N.-C. Ha and E.-h. Jho for providing cells and reagents. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIP) (grants 2016R1A5A1004694, 2015R1A2A1A05001873). Y.-H.C. and S.C. were supported by a BK21 studentship from the NRF.
Author information
Authors and Affiliations
Contributions
P.-H.C., Y.-H.C., S.-K.L., W.-J.J., B.-S.M., J.-H.Y., S.C., J.Y., M.-Y.K. and S.K. designed and performed the all experiments. J.L. and J.S.Y. synthesized chemicals. P.H.C., J.S.Y., H.-Y.K., D.S.M., H.K., W.L. G.H. and K.-Y.C. performed data analysis. P.-H.C., Y.-H.C., J.Y., J.S.Y., D.S.M., G.H. and K.-Y.C. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Figures 1–24 and Supplementary Tables 1–4. (PDF 4185 kb)
Supplementary Note
Synthetic procedures. (PDF 346 kb)
Supplementary Data
Source data for Supplementary Figures 2–7, 9 and 11–14. (XLSX 247 kb)
Rights and permissions
About this article
Cite this article
Cha, PH., Cho, YH., Lee, SK. et al. Small-molecule binding of the axin RGS domain promotes β-catenin and Ras degradation. Nat Chem Biol 12, 593–600 (2016). https://doi.org/10.1038/nchembio.2103
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2103
This article is cited by
-
Inhibition of ADAM9 promotes the selective degradation of KRAS and sensitizes pancreatic cancers to chemotherapy
Nature Cancer (2024)
-
An overview of recent advancements in small molecules suppression of oncogenic signaling of K-RAS: an updated review
Molecular Diversity (2024)
-
Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities
Signal Transduction and Targeted Therapy (2022)
-
Wnt/β-catenin signaling in cancers and targeted therapies
Signal Transduction and Targeted Therapy (2021)
-
APC loss induces Warburg effect via increased PKM2 transcription in colorectal cancer
British Journal of Cancer (2021)