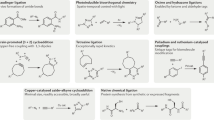
Abstract
Bioorthogonal chemical reactions are a thriving area of chemical research in recent years as an unprecedented technique to dissect native biological processes through chemistry-enabled strategies. However, current concepts of bioorthogonal chemistry have largely centered on 'bond formation' reactions between two mutually reactive bioorthogonal handles. Recently, in a reverse strategy, a collection of 'bond cleavage' reactions has emerged with excellent biocompatibility. These reactions have expanded our bioorthogonal chemistry repertoire, enabling an array of exciting new biological applications that range from the chemically controlled spatial and temporal activation of intracellular proteins and small-molecule drugs to the direct manipulation of intact cells under physiological conditions. Here we highlight the development and applications of these bioorthogonal cleavage reactions. Furthermore, we lay out challenges and propose future directions along this appealing avenue of research.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Prescher, J.A. & Bertozzi, C.R. Chemistry in living systems. Nat. Chem. Biol. 1, 13–21 (2005). A landmark paper describing the fundamental concepts of bioorthogonal chemistry.
Sletten, E.M. & Bertozzi, C.R. Bioorthogonal chemistry: fishing for selectivity in a sea of functionality. Angew. Chem. Int. Ed. Engl. 48, 6974–6998 (2009).
Bertozzi, C.R. A decade of bioorthogonal chemistry. Acc. Chem. Res. 44, 651–653 (2011).
Walsh, C.T., Garneau-Tsodikova, S. & Gatto, G.J. Protein posttranslational modifications: the chemistry of proteome diversifications. Angew. Chem. Int. Ed. Engl. 44, 7342–7372 (2005).
Baker, M. Direct protein control. Nat. Methods 9, 443–447 (2012).
Rakhit, R., Navarro, R. & Wandless, T.J. Chemical biology strategies for posttranslational control of protein function. Chem. Biol. 21, 1238–1252 (2014).
Muñoz, J. & Heck, A.J.R. From the human genome to the human proteome. Angew. Chem. Int. Ed. Engl. 53, 10864–10866 (2014).
Laughlin, S.T., Baskin, J.M., Amacher, S.L. & Bertozzi, C.R. In vivo imaging of membrane-associated glycans in developing zebrafish. Science 320, 664–667 (2008). This work represents an appealing example on how bioorthogonal chemistry can outcompete conventional approaches in studying certain biological questions.
Zhang, G., Zheng, S., Liu, H. & Chen, P.R. Illuminating biological processes through site-specific protein labeling. Chem. Soc. Rev. 44, 3405–3417 (2015).
Lang, K. & Chin, J.W. Bioorthogonal reactions for labeling proteins. ACS Chem. Biol. 9, 16–20 (2014).
Patterson, D.M., Nazarova, L.A. & Prescher, J.A. Finding the right (bioorthogonal) chemistry. ACS Chem. Biol. 9, 592–605 (2014).
McKay, C.S. & Finn, M.G. Click chemistry in complex mixtures: bioorthogonal bioconjugation. Chem. Biol. 21, 1075–1101 (2014).
Ramil, C.P. & Lin, Q. Bioorthogonal chemistry: strategies and recent developments. Chem. Commun. (Camb.) 49, 11007–11022 (2013).
Thirumurugan, P., Matosiuk, D. & Jozwiak, K. Click chemistry for drug development and diverse chemical-biology applications. Chem. Rev. 113, 4905–4979 (2013).
Borrmann, A. & van Hest, J.C.M. Bioorthogonal chemistry in living organisms. Chem. Sci. 5, 2123–2134 (2014).
Yang, M., Li, J. & Chen, P.R. Transition metal-mediated bioorthogonal protein chemistry in living cells. Chem. Soc. Rev. 43, 6511–6526 (2014).
Yang, M. & Chen, P.R. Progress in the bioorthogonal labeling reactions. Acta Chim. Sinensis. 73, 783–792 (2015).
Spicer, C.D. & Davis, B.G. Selective chemical protein modification. Nat. Commun. 5, 4740 (2014).
Dawson, P.E., Muir, T., Clark-Lewis, I. & Kent, S. Synthesis of proteins by native chemical ligation. Science 266, 776–779 (1994).
Griffin, B.A., Adams, S.R. & Tsien, R.Y. Specific covalent labeling of recombinant protein molecules inside live cells. Science 281, 269–272 (1998).
Saxon, E. & Bertozzi, C.R. Cell surface engineering by a modified Staudinger reaction. Science 287, 2007–2010 (2000).
Hang, H.C., Yu, C., Kato, D.L. & Bertozzi, C.R. A metabolic labeling approach toward proteomic analysis of mucin-type O-linked glycosylation. Proc. Natl. Acad. Sci. USA 100, 14846–14851 (2003). This paper applied the Staudinger ligation reaction on living cells and was the first to use the term 'bioorthogonal chemistry'.
Kolb, H.C., Finn, M.G. & Sharpless, K.B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. Engl. 40, 2004–2021 (2001).
Marczewska, J., Koziorowska, J.H. & Anuszewska, E.L. Influence of ascorbic acid on cytotoxic activity of copper and iron ions in vitro. Acta Pol. Pharm. 57, 415–418 (2000).
Macomber, L. & Imlay, J.A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. USA 106, 8344–8349 (2009).
Debets, M.F. et al. Bioconjugation with strained alkenes and alkynes. Acc. Chem. Res. 44, 805–815 (2011).
Sletten, E.M. & Bertozzi, C.R. From mechanism to mouse: a tale of two bioorthogonal reactions. Acc. Chem. Res. 44, 666–676 (2011).
Blackman, M.L., Royzen, M. & Fox, J.M. Tetrazine ligation: fast bioconjugation based on inverse-electron-demand Diels-Alder reactivity. J. Am. Chem. Soc. 130, 13518–13519 (2008). A report of a highly efficient inverse electron-demand Diels-Alder reaction with an unprecedented reaction rate in living systems.
Devaraj, N.K. & Weissleder, R. Biomedical applications of tetrazine cycloadditions. Acc. Chem. Res. 44, 816–827 (2011).
Selvaraj, R. & Fox, J.M. trans-Cyclooctene—a stable, voracious dienophile for bioorthogonal labeling. Curr. Opin. Chem. Biol. 17, 753–760 (2013).
Soriano del Amo, D. et al. Biocompatible copper(I) catalysts for in vivo imaging of glycans. J. Am. Chem. Soc. 132, 16893–16899 (2010).
Yang, M. et al. Biocompatible click chemistry enabled compartment-specific pH measurement inside E. coli. Nat. Commun. 5, 4981 (2014).
Lin, Y.A., Chalker, J.M., Floyd, N., Bernardes, G.J.L. & Davis, B.G. Allyl sulfides are privileged substrates in aqueous cross-metathesis: application to site-selective protein modification. J. Am. Chem. Soc. 130, 9642–9643 (2008).
Chalker, J.M., Wood, C.S.C. & Davis, B.G. A convenient catalyst for aqueous and protein Suzuki-Miyaura cross-coupling. J. Am. Chem. Soc. 131, 16346–16347 (2009). This paper described the development of Pd-catalyzed cross-coupling reaction with biocompatible ligands for protein modifications.
Li, N., Lim, R.K.V., Edwardraja, S. & Lin, Q. Copper-free Sonogashira cross-coupling for functionalization of alkyne-encoded proteins in aqueous medium and in bacterial cells. J. Am. Chem. Soc. 133, 15316–15319 (2011).
Li, J. et al. Ligand-free palladium-mediated site-specific protein labeling inside gram-negative bacterial pathogens. J. Am. Chem. Soc. 135, 7330–7338 (2013).
Chankeshwara, S.V., Indrigo, E. & Bradley, M. Palladium-mediated chemistry in living cells. Curr. Opin. Chem. Biol. 21, 128–135 (2014).
Lim, R.K.V. & Lin, Q. Photoinducible bioorthogonal chemistry: a spatiotemporally controllable tool to visualize and perturb proteins in live cells. Acc. Chem. Res. 44, 828–839 (2011).
Sasmal, P.K., Streu, C.N. & Meggers, E. Metal complex catalysis in living biological systems. Chem. Commun. (Camb.) 49, 1581–1587 (2013).
Völker, T. & Meggers, E. Transition-metal-mediated uncaging in living human cells—an emerging alternative to photolabile protecting groups. Curr. Opin. Chem. Biol. 25, 48–54 (2015).
Klán, P. et al. Photoremovable protecting groups in chemistry and biology: reaction mechanisms and efficacy. Chem. Rev. 113, 119–191 (2013).
Donato, L. et al. Water-soluble, donor-acceptor biphenyl derivatives in the 2-(o-nitrophenyl)propyl series: highly efficient two-photon uncaging of the neurotransmitter γ-aminobutyric acid at l=800 nm. Angew. Chem. Int. Ed. Engl. 51, 1840–1843 (2012).
Xu, J. et al. A rapid response “Turn-On” fluorescent probe for nitroreductase detection and its application in hypoxic tumor cell imaging. Analyst 140, 574–581 (2015).
Bae, J. et al. Nitroreductase-triggered activation of a novel caged fluorescent probe obtained from methylene blue. Chem. Commun. (Camb.) 51, 12787–12790 (2015).
Streu, C. & Meggers, E. Ruthenium-induced allylcarbamate cleavage in living cells. Angew. Chem. Int. Ed. Engl. 45, 5645–5648 (2006). This study is one of the earliest examples of chemically mediated biocompatible cleavage reactions on small molecules inside living cells.
Völker, T., Dempwolff, F., Graumann, P.L. & Meggers, E. Progress towards bioorthogonal catalysis with organometallic compounds. Angew. Chem. Int. Ed. Engl. 53, 10536–10540 (2014).
Isidro-Llobet, A., Álvarez, M. & Albericio, F. Amino acid-protecting groups. Chem. Rev. 109, 2455–2504 (2009).
Yusop, R.M., Unciti-Broceta, A., Johansson, E.M.V., Sánchez-Martín, R.M. & Bradley, M. Palladium-mediated intracellular chemistry. Nat. Chem. 3, 239–243 (2011). This work first showed that palladium-mediated reactions could be employed inside living mammalian cells.
Li, J. et al. Palladium-triggered deprotection chemistry for protein activation in living cells. Nat. Chem. 6, 352–361 (2014). This study demonstrated that Pd-mediated bioorthogonal cleavage chemistry could be used to activate a protein of interest in living cells.
Weiss, J.T. et al. Development and bioorthogonal activation of palladium-labile prodrugs of gemcitabine. J. Med. Chem. 57, 5395–5404 (2014).
Kislukhin, A.A., Hong, V.P., Breitenkamp, K.E. & Finn, M.G. Relative performance of alkynes in copper-catalyzed azide-alkyne cycloaddition. Bioconjug. Chem. 24, 684–689 (2013).
Versteegen, R.M., Rossin, R., ten Hoeve, W., Janssen, H.M. & Robillard, M.S. Click to release: instantaneous doxorubicin elimination upon tetrazine ligation. Angew. Chem. Int. Ed. Engl. 52, 14112–14116 (2013).
Li, J., Jia, S. & Chen, P.R. Diels-Alder reaction–triggered bioorthogonal protein decaging in living cells. Nat. Chem. Biol. 10, 1003–1005 (2014). This work demonstrated a highly efficient bioorthogonal cleavage chemistry for protein activation in living systems.
Matikonda, S.S. et al. Bioorthogonal prodrug activation driven by a strain-promoted 1,3-dipolar cycloaddition. Chem. Sci. 6, 1212–1218 (2015).
Pawlak, J.B. et al. Bioorthogonal deprotection on the dendritic cell surface for chemical control of antigen cross-presentation. Angew. Chem. Int. Ed. Engl. 54, 5628–5631 (2015). This study utilized a bioorthogonal cleavage reactions to manipulate T cell activation.
Chen, Y., Kamlet, A.S., Steinman, J.B. & Liu, D.R. A biomolecule-compatible visible-light-induced azide reduction from a DNA-encoded reaction-discovery system. Nat. Chem. 3, 146–153 (2011).
Hu, C. & Chen, Y. Biomolecule-compatible chemical bond-formation and bond-cleavage reactions induced by visible light. Tetrahedr. Lett. 56, 884–888 (2015).
Li, L. et al. A sensitive two-photon probe to selectively detect monoamine oxidase B activity in Parkinson's disease models. Nat. Commun. 5, 3276 (2014).
Ritter, C. et al. Bioorthogonal enzymatic activation of caged compounds. Angew. Chem. Int. Ed. Engl. 54, 13440–13443 (2015). This work developed a potentially powerful bioorthogonal enzymatic cleavage reaction through directed evolution.
Tian, L. et al. Selective esterase-ester pair for targeting small molecules with cellular specificity. Proc. Natl. Acad. Sci. USA 109, 4756–4761 (2012).
Marletta, M.A. Raising enzymes from the dead and the secrets they can tell. ACS Chem. Biol. 1, 73–74 (2006).
Zorn, J.A. & Wells, J.A. Turning enzymes ON with small molecules. Nat. Chem. Biol. 6, 179–188 (2010).
Qiao, Y., Molina, H., Pandey, A., Zhang, J. & Cole, P.A. Chemical rescue of a mutant enzyme in living cells. Science 311, 1293–1297 (2006).
Armbruster, B.N., Li, X., Pausch, M.H., Herlitze, S. & Roth, B.L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. USA 104, 5163–5168 (2007).
Baker, A.S. & Deiters, A. Optical control of protein function through unnatural amino acid mutagenesis and other optogenetic approaches. ACS Chem. Biol. 9, 1398–1407 (2014).
Chin, J.W. Expanding and reprogramming the genetic code of cells and animals. Annu. Rev. Biochem. 83, 379–408 (2014).
Liu, C.C. & Schultz, P.G. Adding new chemistries to the genetic code. Annu. Rev. Biochem. 79, 413–444 (2010).
Wan, W., Tharp, J.M. & Liu, W.R. Pyrrolysyl-tRNA synthetase: an ordinary enzyme but an outstanding genetic code expansion tool. Biochim. Biophys. Acta 1844, 1059–1070 (2014).
Cui, J. & Shao, F. Biochemistry and cell signaling taught by bacterial effectors. Trends Biochem. Sci. 36, 532–540 (2011).
Li, H. et al. The phosphothreonine lyase activity of a bacterial Type III effector family. Science 315, 1000–1003 (2007).
Gautier, A., Deiters, A. & Chin, J.W. Light-activated kinases enable temporal dissection of signaling networks in living cells. J. Am. Chem. Soc. 133, 2124–2127 (2011). This study combined photo-decaging reaction with genetic code expansion technique for selective activation of a specific kinase in living cells.
Manning, G., Whyte, D.B., Martinez, R., Hunter, T. & Sudarsanam, S. The protein kinase complement of the human genome. Science 298, 1912–1934 (2002).
Schmelz, S. & Naismith, J.H. Adenylate-forming enzymes. Curr. Opin. Struct. Biol. 19, 666–671 (2009).
Wang, J. et al. Chemical remodeling of cell-surface sialic acids through a palladium-triggered bioorthogonal elimination reaction. Angew. Chem. Int. Ed. Engl. 54, 5364–5368 (2015).
Huttunen, K.M., Raunio, H. & Rautio, J. Prodrugs—from serendipity to rational design. Pharmacol. Rev. 63, 750–771 (2011).
Mahato, R., Tai, W. & Cheng, K. Prodrugs for improving tumor targetability and efficiency. Adv. Drug Deliv. Rev. 63, 659–670 (2011).
Weiss, J.T. et al. Extracellular palladium-catalysed dealkylation of 5-fluoro-1-propargyl-uracil as a bioorthogonally activated prodrug approach. Nat. Commun. 5, 3277 (2014). This paper demonstrated the novel concept of bioorthogonal prodrug activation through Pd-mediated deprotection.
Tonga, G.Y. et al. Supramolecular regulation of bioorthogonal catalysis in cells using nanoparticle-embedded transition metal catalysts. Nat. Chem. 7, 597–603 (2015).
Chan, J., Dodani, S.C. & Chang, C.J. Reaction-based small-molecule fluorescent probes for chemoselective bioimaging. Nat. Chem. 4, 973–984 (2012).
Garner, A.L. & Koide, K. Studies of a fluorogenic probe for palladium and platinum leading to a palladium-specific detection method. Chem. Comm., 86–88 (2009).
Santra, M., Ko, S.-K., Shin, I. & Ahn, K.H. Fluorescent detection of palladium species with an O-propargylated fluorescein. Chem. Commun. (Camb.) 46, 3964–3966 (2010).
Ke, B. et al. A fluorescent probe for rapid aqueous fluoride detection and cell imaging. Chem. Commun. (Camb.) 49, 2494–2496 (2013).
Roy, A., Datar, A., Kand, D., Saha, T. & Talukdar, P. A fluorescent off-on NBD-probe for F-sensing: theoretical validation and experimental studies. Org. Biomol. Chem. 12, 2143–2149 (2014).
Sachdeva, A., Wang, K., Elliott, T. & Chin, J.W. Concerted, rapid, quantitative, and site-specific dual labeling of proteins. J. Am. Chem. Soc. 136, 7785–7788 (2014).
Li, Q., Dong, T., Liu, X. & Lei, X. A bioorthogonal ligation enabled by click cycloaddition of o-quinolinone quinone methide and vinyl thioether. J. Am. Chem. Soc. 135, 4996–4999 (2013).
Nikić, I. et al. Minimal tags for rapid dual-color live-cell labeling and super-resolution microscopy. Angew. Chem. Int. Ed. Engl. 53, 2245–2249 (2014).
Zhang, X. et al. Second generation TQ-ligation for cell organelle imaging. ACS Chem. Biol. 10, 1676–1683 (2015).
Karginov, A.V. et al. Dissecting motility signaling through activation of specific Src-effector complexes. Nat. Chem. Biol. 10, 286–290 (2014).
Chu, P.-H. et al. Engineered kinase activation reveals unique morphodynamic phenotypes and associated trafficking for Src family isoforms. Proc. Natl. Acad. Sci. USA 111, 12420–12425 (2014).
Valencia, A., Chardin, P., Wittinghofer, A. & Sander, C. The ras protein family: evolutionary tree and role of conserved amino acids. Biochemistry 30, 4637–4648 (1991).
Jiang, J., Lazarus, M.B., Pasquina, L., Sliz, P. & Walker, S. A neutral diphosphate mimic crosslinks the active site of human O-GlcNAc transferase. Nat. Chem. Biol. 8, 72–77 (2012).
Patricelli, M.P. & Cravatt, B.F. Clarifying the catalytic roles of conserved residues in the amidase signature family. J. Biol. Chem. 275, 19177–19184 (2000).
Weiss, J.T., Carragher, N.O. & Unciti-Broceta, A. Palladium-mediated dealkylation of N-propargyl-floxuridine as a bioorthogonal oxygen-independent prodrug strategy. Sci. Rep. 5, 9329 (2015).
Chari, R.V.J., Miller, M.L. & Widdison, W.C. Antibody-drug conjugates: an emerging concept in cancer therapy. Angew. Chem. Int. Ed. Engl. 53, 3796–3827 (2014).
Kim, J. & Bertozzi, C.R. A bioorthogonal reaction of N-oxide and boron reagents. Angew. Chem. Int. Ed. Engl. 54, 15777–15781 (2015). This study, published while our manuscript is under review, reported another new type of bioorthogonal bond-cleavage reaction between N-oxide and boron reagents.
Acknowledgements
Our research interests in expanding the bioorthogonal chemistry toolkit have been generously supported by the National Natural Science Foundation of China (21225206, 21521003 and 21432002) and the National Basic Research Program of China (2012CB917301).
Author information
Authors and Affiliations
Contributions
P.R.C. and J.L. wrote the manuscript and prepared the figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Li, J., Chen, P. Development and application of bond cleavage reactions in bioorthogonal chemistry. Nat Chem Biol 12, 129–137 (2016). https://doi.org/10.1038/nchembio.2024
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2024
This article is cited by
-
An all-in-one tetrazine reagent for cysteine-selective labeling and bioorthogonal activable prodrug construction
Nature Communications (2024)
-
Lighting up kinase contacts in situ
Nature Chemical Biology (2024)
-
Bioorthogonal chemistry based on-demand drug delivery system in cancer therapy
Frontiers of Chemical Science and Engineering (2023)
-
Recent advances in developing active targeting and multi-functional drug delivery systems via bioorthogonal chemistry
Signal Transduction and Targeted Therapy (2022)
-
Nanovoid-confinement and click-activated nanoreactor for synchronous delivery of prodrug pairs and precise photodynamic therapy
Nano Research (2022)