Abstract
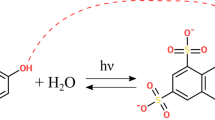
We genetically encoded the photocaged amino acid 4,5-dimethoxy-2-nitrobenzylserine (DMNB-Ser, 1) in Saccharomyces cerevisiae in response to the amber nonsense codon TAG. This amino acid was converted to serine in living cells by irradiation with relatively low-energy blue light and was used to noninvasively photoactivate phosphorylation of the transcription factor Pho4, which controls the cellular response to inorganic phosphate1. When substituted at phosphoserine sites that control nuclear export of Pho4, 1 blocks phosphorylation and subsequent export by the receptor Msn5 (ref. 2). We triggered phosphorylation of individual serine residues with a visible laser pulse and monitored nuclear export of Pho4-GFP fusion constructs in real time. We observed distinct export kinetics for differentially phosphorylated Pho4 mutants, which demonstrates dynamic regulation of Pho4 function. This methodology should also facilitate the analysis of other cellular processes involving free serine residues, including catalysis, biomolecular recognition and ion transport.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
O'Neill, E.M., Kaffman, A., Jolly, E.R. & O'Shea, E.K. Regulation of PHO4 nuclear localization by the PHO80–PHO85 cyclin-CDK complex. Science 271, 209–212 (1996).
Kaffman, A., Rank, N.M., O'Neill, E.M., Huang, L.S. & O'Shea, E.K. The receptor Msn5 exports the phosphorylated transcription factor Pho4 out of the nucleus. Nature 396, 482–486 (1998).
Pelliccioli, A.P. & Wirz, J. Photoremovable protecting groups: reaction mechanisms and applications. Photochem. Photobiol. Sci. 1, 441–458 (2002).
Mayer, G. & Heckel, A. Biologically active molecules with a “light switch”. Angew. Chem. Int. Edn Engl. 45, 4900–4921 (2006).
Lawrence, D.S. The preparation and in vivo applications of caged peptides and proteins. Curr. Opin. Chem. Biol. 9, 570–575 (2005).
Rothman, D.M., Shults, M.D. & Imperiali, B. Chemical approaches for investigating phosphorylation in signal transduction networks. Trends Cell Biol. 15, 502–510 (2005).
Ghosh, M., Ichetovkin, I., Song, X., Condeelis, J.S. & Lawrence, D.S. A new strategy for caging proteins regulated by kinases. J. Am. Chem. Soc. 124, 2440–2441 (2002).
Zou, K., Cheley, S., Givens, R.S. & Bayley, H. Catalytic subunit of protein kinase A caged at the activating phosphothreonine. J. Am. Chem. Soc. 124, 8220–8229 (2002).
Endo, M., Nakayama, K., Kaida, Y. & Majima, T. Design and synthesis of photochemically controllable caspase-3. Angew. Chem. Int. Edn. Engl. 43, 5643–5645 (2004).
Hahn, M.E. & Muir, T.W. Photocontrol of Smad2, a multiphosphorylated cell-signaling protein, through caging of activating phosphoserines. Angew. Chem. Int. Edn. Engl. 43, 5800–5803 (2004).
England, P.M., Lester, H.A., Davidson, N. & Dougherty, D.A. Site-specific, photochemical proteolysis applied to ion channels in vivo. Proc. Natl. Acad. Sci. USA 94, 11025–11030 (1997).
Volgraf, M. et al. Allosteric control of an ionotropic glutamate receptor with an optical switch. Nat. Chem. Biol. 2, 47–52 (2006).
Banghart, M., Borges, K., Isacoff, E., Trauner, D. & Kramer, R.H. Light-activated ion channels for remote control of neuronal firing. Nat. Neurosci. 7, 1381–1386 (2004).
Deiters, A., Groff, D., Ryu, Y., Xie, J. & Schultz, P.G. A genetically encoded photocaged tyrosine. Angew. Chem. Int. Edn Engl. 45, 2728–2731 (2006).
Wu, N., Deiters, A., Cropp, T.A., King, D. & Schultz, P.G. A genetically encoded photocaged amino acid. J. Am. Chem. Soc. 126, 14306–14307 (2004).
Walker, J.W., Martin, H., Schmitt, F.R. & Barsotti, R.J. Rapid release of an alpha-adrenergic receptor ligand from photolabile analogues. Biochemistry 32, 1338–1345 (1993).
Cusack, S., Yaremchuk, A. & Tukalo, M. The 2 A crystal structure of leucyl-tRNA synthetase and its complex with a leucyl-adenylate analogue. EMBO J. 19, 2351–2361 (2000).
Summerer, D. et al. A genetically encoded fluorescent amino acid. Proc. Natl. Acad. Sci. USA 103, 9785–9789 (2006).
Xie, J. & Schultz, P.G. A chemical toolkit for proteins–an expanded genetic code. Nat. Rev. Mol. Cell Biol. 7, 775–782 (2006).
Mursinna, R.S. & Martinis, S.A. Rational design to block amino acid editing of a tRNA synthetase. J. Am. Chem. Soc. 124, 7286–7287 (2002).
Lincecum, T.L., Jr. et al. Structural and mechanistic basis of pre- and posttransfer editing by leucyl-tRNA synthetase. Mol. Cell 11, 951–963 (2003).
Oshima, Y. The phosphatase system in Saccharomyces cerevisiae. Genes Genet. Syst. 72, 323–334 (1997).
Komeili, A. & O'Shea, E.K. Roles of phosphorylation sites in regulating activity of the transcription factor Pho4. Science 284, 977–980 (1999).
Jeffery, D.A., Springer, M., King, D.S. & O'Shea, E.K. Multi-site phosphorylation of Pho4 by the cyclin-CDK Pho80-Pho85 is semi-processive with site preference. J. Mol. Biol. 306, 997–1010 (2001).
Springer, M., Wykoff, D.D., Miller, N. & O'Shea, E.K. Partially phosphorylated Pho4 activates transcription of a subset of phosphate-responsive genes. PLoS Biol. 1, E28 (2003).
Kaffman, A., Rank, N.M. & O'Shea, E.K. Phosphorylation regulates association of the transcription factor Pho4 with its import receptor Pse1/Kap121. Genes Dev. 12, 2673–2683 (1998).
Olivo-Marin, J.C. Extraction of spots in biological images using multiscale products. Pattern Recognit. 35, 1989–1996 (2002).
Thomson, D.J. & Chave, A.D. in Advances in Spectrum Estimation (ed. Haykin, S.) 58–113 (Prentice Hall, Englewood Cliffs, New Jersey, USA, 1991).
Veldhuyzen, W.F., Nguyen, Q., McMaster, G. & Lawrence, D.S.A. Light-activated probe of intracellular protein kinase activity. J. Am. Chem. Soc. 125, 13358–13359 (2003).
Acknowledgements
We thank E. O'Shea (Howard Hughes Medical Institute, Harvard University) for the PRS-Pho4WT-GFP plasmid, the mass spectrometry facility of the Genomics Institute of the Novartis Research Foundation for protein mass measurements and E. Peters for helpful discussions. E.A.L. and D.S. acknowledge a postdoctoral scholarship from the Alexander von Humboldt Foundation. This work was also supported by the US Department of Energy (DE-FG03-00ER46051) and the Skaggs Institute for Chemical Biology.
Author information
Authors and Affiliations
Contributions
E.A.L. designed experiments, performed biological experiments, imaging and data analysis, and wrote the manuscript; D.S. designed experiments, evolved the synthetase, performed biological experiments and wrote the manuscript; B.H.G. and S.M.B. provided mass spectrometric analysis; B.H.G. assisted in editing the manuscript; P.G.S. designed experiments and wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 217 kb)
Rights and permissions
About this article
Cite this article
Lemke, E., Summerer, D., Geierstanger, B. et al. Control of protein phosphorylation with a genetically encoded photocaged amino acid. Nat Chem Biol 3, 769–772 (2007). https://doi.org/10.1038/nchembio.2007.44
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2007.44
This article is cited by
-
Sequence-based prediction of the intrinsic solubility of peptides containing non-natural amino acids
Nature Communications (2023)
-
Remodeling the cellular stress response for enhanced genetic code expansion in mammalian cells
Nature Communications (2023)
-
Dual photo-controlled release system for fipronil and dinotefuran
Photochemical & Photobiological Sciences (2022)
-
Expanding the enzyme universe with genetically encoded unnatural amino acids
Nature Catalysis (2020)
-
The light-sensitive dimerizer zapalog reveals distinct modes of immobilization for axonal mitochondria
Nature Cell Biology (2019)