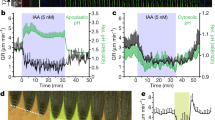
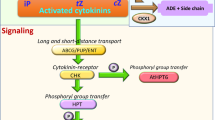
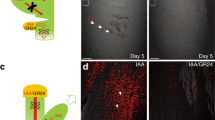
Abstract
As multicellular organisms, plants, like animals, use endogenous signaling molecules to coordinate their own physiology and development. To compensate for the absence of a cardiovascular system, plants have evolved specialized transport pathways to distribute signals and nutrients. The main transport streams include the xylem flow of the nutrients from the root to the shoot and the phloem flow of materials from the photosynthetic active tissues. These long-distance transport processes are complemented by several intercellular transport mechanisms (apoplastic, symplastic and transcellular transport). A prominent example of transcellular flow is transport of the phytohormone auxin within tissues. The process is mediated by influx and efflux carriers, whose polar localization in the plasma membrane determines the directionality of the flow. This polar auxin transport generates auxin maxima and gradients within tissues that are instrumental in the diverse regulation of various plant developmental processes, including embryogenesis, organogenesis, vascular tissue formation and tropisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Valladares, F., Gianoli, E. & Gómez, J. Ecological limits to plant phenotypic plasticity. New Phytol. 176, 749–763 (2007).
Alabadí, D. & Blázquez, M.A. Molecular interactions between light and hormone signaling to control plant growth. Plant Mol. Biol. 69, 409–417 (2009).
Kim, G.T., Yano, S., Kozuka, T. & Tsukaya, H. Photomorphogenesis of leaves: shade-avoidance and differentiation of sun and shade leaves. Photochem. Photobiol. Sci. 4, 770–774 (2005).
Esmon, C.A., Pedmale, U.V. & Liscum, E. Plant tropisms: providing the power of movement to a sessile organism. Int. J. Dev. Biol. 49, 665–674 (2005).
Gomez-Roldan, V. et al. Strigolactone inhibition of shoot branching. Nature 455, 189–194 (2008).
Umehara, M. et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 455, 195–200 (2008).
Bishopp, A., Mähönen, A.P. & Helariutta, Y. Signs of change: hormone receptors that regulate plant development. Development 133, 1857–1869 (2006).
Berger, S. Jasmonate-related mutants of Arabidopsis as tools for studying stress signaling. Planta 214, 497–504 (2002).
Spoel, S.H. & Dong, X. Making sense of hormone crosstalk during plant immune responses. Cell Host Microbe 3, 348–351 (2008).
Aloni, R., Aloni, E., Langhans, M. & Ullrich, C. Role of auxin in regulating Arabidopsis flower development. Planta 223, 315–328 (2006).
Cecchetti, V., Altamura, M.M., Falasca, G., Costantino, P. & Cardarelli, M. Auxin regulates Arabidopsis anther dehiscence, pollen maturation, and filament elongation. Plant Cell 20, 1760–1774 (2008).
Friml, J. et al. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426, 147–153 (2003).
Reinhardt, D. Vascular patterning: more than just auxin? Curr. Biol. 13, R485–R487 (2003).
Reinhardt, D. Phyllotaxis—a new chapter in an old tale about beauty and magic numbers. Curr. Opin. Plant Biol. 8, 487–493 (2005).
Benková, E. et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115, 591–602 (2003).
Dinneny, J.R. & Yanofsky, M.F. Vascular patterning: xylem or phloem? Curr. Biol. 14, R112–R114 (2004).
Ding, B., Itaya, A. & Qi, Y. Symplasmic protein and RNA traffic: regulatory points and regulatory factors. Curr. Opin. Plant Biol. 6, 596–602 (2003).
Maule, A.J. Plasmodesmata: structure, function and biogenesis. Curr. Opin. Plant Biol. 11, 680–686 (2008).
Hose, E., Clarkson, D.T., Steudle, E., Schreiber, L. & Hartung, W. The exodermis: a variable apoplastic barrier. J. Exp. Bot. 52, 2245–2264 (2001).
Amtmann, A. & Blatt, M.R. Regulation of macronutrient transport. New Phytol. 181, 35–52 (2009).
Clapham, D.E. Calcium signaling. Cell 131, 1047–1058 (2007).
Scofield, G.N. et al. The role of the sucrose transporter, OsSUT1, in germination and early seedling growth and development of rice plants. J. Exp. Bot. 58, 483–495 (2007).
Lucas, W.J. Plant viral movement proteins: agents for cell-to-cell trafficking of viral genomes. Virology 344, 169–184 (2006).
Kankanala, P., Czymmek, K. & Valent, B. Roles for rice membrane dynamics and plasmodesmata during biotrophic invasion by the blast fungus. Plant Cell 19, 706–724 (2007).
Jiang, F. & Hartung, W. Long-distance signalling of abscisic acid (ABA): the factors regulating the intensity of the ABA signal. J. Exp. Bot. 59, 37–43 (2008).
Hirose, N. et al. Regulation of cytokinin biosynthesis, compartmentalization and translocation. J. Exp. Bot. 59, 75–83 (2008).
Booker, J. et al. MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev. Cell 8, 443–449 (2005).
Cambridge, A.P. & Morris, D.A. Transfer of exogenous auxin from the phloem to the polar auxin transport pathway in pea (Pisum sativum L.). Planta 199, 583–588 (1996).
Lough, T.J. & Lucas, W.J. Integrative plant biology: role of phloem long-distance macromolecular trafficking. Annu. Rev. Plant Biol. 57, 203–232 (2006).
Giakountis, A. & Coupland, G. Phloem transport of flowering signals. Curr. Opin. Plant Biol. 11, 687–694 (2008).
Fukuda, H. Signals that control plant vascular cell differentiation. Nat. Rev. Mol. Cell Biol. 5, 379–391 (2004).
Sauer, M. et al. Canalization of auxin flow by Aux/IAA-ARF-dependent feedback regulation of PIN polarity. Genes Dev. 20, 2902–2911 (2006).
Sachs, T. Integrating cellular and organismic aspects of vascular differentiation. Plant Cell Physiol. 41, 649–656 (2000).
Mähönen, A.P. et al. Cytokinin signaling and its inhibitor AHP6 regulate cell fate during vascular development. Science 311, 94–98 (2006).
Choe, S. et al. The Arabidopsis dwf7/ste1 mutant is defective in the delta7 sterol C-5 desaturation step leading to brassinosteroid biosynthesis. Plant Cell 11, 207–221 (1999).
Tester, M. & Leigh, R.A. Partitioning of nutrient transport processes in roots. J. Exp. Bot. 52, 445–457 (2001).
Zimmermann, H.M., Hartmann, K., Schreiber, L. & Steudle, E. Chemical composition of apoplastic transport barriers in relation to radial hydraulic conductivity of corn roots (Zea mays L.). Planta 210, 302–311 (2000).
Symons, G.M., Ross, J.J., Jager, C.E. & Reid, J.B. Brassinosteroid transport. J. Exp. Bot. 59, 17–24 (2008).
Rojo, E., Sharma, V.K., Kovaleva, V., Raikhel, N.V. & Fletcher, J.C. CLV3 is localized to the extracellular space, where it activates the Arabidopsis CLAVATA stem cell signaling pathway. Plant Cell 14, 969–977 (2002).
Kim, I., Cho, E., Crawford, K., Hempel, F.D. & Zambryski, P.C. Cell-to-cell movement of GFP during embryogenesis and early seedling development in Arabidopsis. Proc. Natl. Acad. Sci. USA 102, 2227–2231 (2005).
Kim, I., Kobayashi, K., Cho, E. & Zambryski, P.C. Subdomains for transport via plasmodesmata corresponding to the apical-basal axis are established during Arabidopsis embryogenesis. Proc. Natl. Acad. Sci. USA 102, 11945–11950 (2005).
Sauer, N. Molecular physiology of higher plant sucrose transporters. FEBS Lett. 581, 2309–2317 (2007).
Dunoyer, P., Himber, C., Ruiz-Ferrer, V., Alioua, A. & Voinnet, O. Intra- and intercellular RNA interference in Arabidopsis thaliana requires components of the microRNA and heterochromatic silencing pathways. Nat. Genet. 39, 848–856 (2007).
Kurata, T. et al. Cell-to-cell movement of the CAPRICE protein in Arabidopsis root epidermal cell differentiation. Development 132, 5387–5398 (2005).
Lee, J.Y. et al. Transcriptional and posttranscriptional regulation of transcription factor expression in Arabidopsis roots. Proc. Natl. Acad. Sci. USA 103, 6055–6060 (2006).
Lucas, W.J. et al. Selective trafficking of KNOTTED1 homeodomain protein and its mRNA through plasmodesmata. Science 270, 1980–1983 (1995).
Nakajima, K., Sena, G., Nawy, T. & Benfey, P.N. Intercellular movement of the putative transcription factor SHR in root patterning. Nature 413, 307–311 (2001).
Crawford, K.M. & Zambryski, P.C. Non-targeted and targeted protein movement through plasmodesmata in leaves in different developmental and physiological states. Plant Physiol. 125, 1802–1812 (2001).
Buchner, P., Takahashi, H. & Hawkesford, M.J. Plant sulphate transporters: co-ordination of uptake, intracellular and long-distance transport. J. Exp. Bot. 55, 1765–1773 (2004).
Loqué, D. & von Wirén, N. Regulatory levels for the transport of ammonium in plant roots. J. Exp. Bot. 55, 1293–1305 (2004).
Fujii, H., Chiou, T.J., Lin, S.I., Aung, K. & Zhu, J. A miRNA involved in phosphate-starvation response in Arabidopsis. Curr. Biol. 15, 2038–2043 (2005).
Peng, M., Hannam, C., Gu, H., Bi, Y.M. & Rothstein, S.J. A mutation in NLA, which encodes a RING-type ubiquitin ligase, disrupts the adaptability of Arabidopsis to nitrogen limitation. Plant J. 50, 320–337 (2007).
Takano, J., Miwa, K. & Fujiwara, T. Boron transport mechanisms: collaboration of channels and transporters. Trends Plant Sci. 13, 451–457 (2008).
Miller, A.J., Fan, X., Shen, Q. & Smith, S.J. Amino acids and nitrate as signals for the regulation of nitrogen acquisition. J. Exp. Bot. 59, 111–119 (2008).
Kasai, M. Regulation of leaf photosynthetic rate correlating with leaf carbohydrate status and activation state of Rubisco under a variety of photosynthetic source/sink balances. Physiol. Plant. 134, 216–226 (2008).
Paul, M.J. & Pellny, T.K. Carbon metabolite feedback regulation of leaf photosynthesis and development. J. Exp. Bot. 54, 539–547 (2003).
Camacho-Cristóbal, J.J. & González-Fontes, A. Boron deficiency decreases plasmalemma H+-ATPase expression and nitrate uptake, and promotes ammonium assimilation into asparagine in tobacco roots. Planta 226, 443–451 (2007).
Mérigout, P. et al. Physiological and transcriptomic aspects of urea uptake and assimilation in Arabidopsis plants. Plant Physiol. 147, 1225–1238 (2008).
Takano, J. et al. The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell 18, 1498–1509 (2006).
Tanaka, M., Wallace, I.S., Takano, J., Roberts, D.M. & Fujiwara, T. NIP6;1 is a boric acid channel for preferential transport of boron to growing shoot tissues in Arabidopsis. Plant Cell 20, 2860–2875 (2008).
Miwa, K. et al. Plants tolerant of high boron levels. Science 318, 1417 (2007).
Darwin, C. The Power of Movement in Plants (John Murray, London, 1880).
Went, F.W. & Thimann, K.V. Phytohormones (Macmillan, New York, 1937).
Ulmasov, T., Murfett, J., Hagen, G. & Guilfoyle, T.J. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9, 1963–1971 (1997).
Dubrovsky, J.G. et al. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc. Natl. Acad. Sci. USA 105, 8790–8794 (2008).
Heisler, M.G. et al. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the inflorescence meristem. Curr. Biol. 15, 1899–1911 (2005).
Nemhauser, J.L., Feldman, L.J. & Zambryski, P.C. Auxin and ETTIN in Arabidopsis gynoecium morphogenesis. Development 127, 3877–3888 (2000).
Sabatini, S. et al. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99, 463–472 (1999).
Friml, J. et al. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell 108, 661–673 (2002).
Sorefan, K. et al. A regulated auxin minimum is required for seed dispersal in Arabidopsis. Nature advance online publication, doi:10.1038/nature07875 (2009).
Mockaitis, K. & Estelle, M. Auxin receptors and plant development: a new signaling paradigm. Annu. Rev. Cell Dev. Biol. 24, 55–80 (2008).
Friml, J. et al. A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science 306, 862–865 (2004).
Cheng, Y., Dai, X. & Zhao, Y. Auxin synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis. Plant Cell 19, 2430–2439 (2007).
Cheng, Y., Dai, X. & Zhao, Y. Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev. 20, 1790–1799 (2006).
Stepanova, A.N. et al. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133, 177–191 (2008).
Vanneste, S., & Friml, J. Auxin: a trigger for change in plant development. Cell 136, 1005–1016 (2009).
Rubery, P.H. & Sheldrake, A.R. Carrier-mediated auxin transport. Planta 118, 101–121 (1974).
Raven, J. Transport of indolacetic acid in plant cells in relation to pH and electrical potential gradients, and its ignificaance for polar IAA transport. New Phytol. 74, 163–172 (1975).
Friml, J., Wiśniewska, J., Benková, E., Mendgen, K. & Palme, K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415, 806–809 (2002).
Gälweiler, L. et al. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282, 2226–2230 (1998).
Müller, A. et al. AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 17, 6903–6911 (1998).
Wis´niewska, J. et al. Polar PIN localization directs auxin flow in plants. Science 312, 858–860 (2006).
Bennett, M.J. et al. Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science 273, 948–950 (1996).
Yang, Y., Hammes, U.Z., Taylor, C.G., Schachtman, D.P. & Nielsen, E. High-affinity auxin transport by the AUX1 influx carrier protein. Curr. Biol. 16, 1123–1127 (2006).
Swarup, R. et al. Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev. 15, 2648–2653 (2001).
Bainbridge, K. et al. Auxin influx carriers stabilize phyllotactic patterning. Genes Dev. 22, 810–823 (2008).
Swarup, K. et al. The auxin influx carrier LAX3 promotes lateral root emergence. Nat. Cell Biol. 10, 946–954 (2008).
Petrášek, J. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312, 914–918 (2006).
Geisler, M. et al. Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J. 44, 179–194 (2005).
Cho, M., Lee, S.H. & Cho, H.-T. P-glycoprotein4 displays auxin efflux transporter-like action in Arabidopsis root hair cells and tobacco cells. Plant Cell 19, 3930–3943 (2007).
Mravec, J. et al. Interaction of PIN and PGP transport mechanisms in auxin distribution-dependent development. Development 135, 3345–3354 (2008).
Blakeslee, J.J. et al. Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. Plant Cell 19, 131–147 (2007).
Titapiwatanakun, B. et al. ABCB19/PGP19 stabilises PIN1 in membrane microdomains in Arabidopsis. Plant J. 57, 27–44 (2009).
Kleine-Vehn, J. & Friml, J. Polar targeting and endocytic recycling in auxin-dependent plant development. Annu. Rev. Cell Dev. Biol. 24, 447–473 (2008).
Tanaka, H., Dhonukshe, P., Brewer, P. & Friml, J. Spatiotemporal asymmetric auxin distribution: a means to coordinate plant development. Cell. Mol. Life Sci. 63, 2738–2754 (2006).
Michniewicz, M. et al. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 130, 1044–1056 (2007).
Dhonukshe, P. et al. Generation of cell polarity in plants links endocytosis, auxin distribution and cell fate decisions. Nature 456, 962–966 (2008).
Harrison, B.R. & Masson, P. ARL2, ARG1 and PIN3 define a gravity signal transduction pathway in root statocytes. Plant J. 53, 380–392 (2008).
Abas, L. et al. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat. Cell Biol. 8, 249–256 (2006).
McCormick, A.J., Cramer, M.D. & Watt, D.A. Changes in photosynthetic rates and gene expression of leaves during a source-sink perturbation in sugarcane. Ann. Bot. (Lond.) 101, 89–102 (2008).
Acknowledgements
We thank E. Meyerowitz for providing seeds of the DR5rev:N7:VENUS, S. Vanneste for providing material for Figure 5 and M. De Cock for help in preparing the manuscript. The authors are supported by the FWO (Fonds voor Wetenschappelijk Onderzoek).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Robert, H., Friml, J. Auxin and other signals on the move in plants. Nat Chem Biol 5, 325–332 (2009). https://doi.org/10.1038/nchembio.170
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.170
This article is cited by
-
Transcriptional analysis reveals formation of axillary solitary hook in vine plant Uncaria rhynchophylla
Plant Biotechnology Reports (2023)
-
The action of enhancing weak light capture via phototropic growth and chloroplast movement in plants
Stress Biology (2022)
-
Effect of macronutrients and micronutrients on biochemical properties in Paulownia shantung
Plant Cell, Tissue and Organ Culture (PCTOC) (2022)
-
Anatomical Modifications Modulated by Pretreatment with 24-Epibrassinolide Alleviate Boron Stress in Soybean Plants: Valuable Repercussions on Nutrient Contents, Photosynthesis, and Biomass
Journal of Soil Science and Plant Nutrition (2022)
-
Manganese toxicity disrupts indole acetic acid homeostasis and suppresses the CO2 assimilation reaction in rice leaves
Scientific Reports (2021)