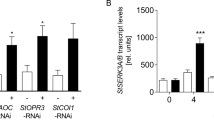
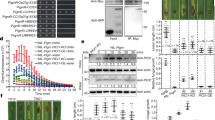
Abstract
Specific chemicals can prime the plant immune system for augmented defense. β-aminobutyric acid (BABA) is a priming agent that provides broad-spectrum disease protection. However, BABA also suppresses plant growth when applied in high doses, which has hampered its application as a crop defense activator. Here we describe a mutant of Arabidopsis thaliana that is impaired in BABA-induced disease immunity (ibi1) but is hypersensitive to BABA-induced growth repression. IBI1 encodes an aspartyl-tRNA synthetase. Enantiomer-specific binding of the R enantiomer of BABA to IBI1 primed the protein for noncanonical defense signaling in the cytoplasm after pathogen attack. This priming was associated with aspartic acid accumulation and tRNA-induced phosphorylation of translation initiation factor eIF2α. However, mutation of eIF2α-phosphorylating GCN2 kinase did not affect BABA-induced immunity but relieved BABA-induced growth repression. Hence, BABA-activated IBI1 controls plant immunity and growth via separate pathways. Our results open new opportunities to separate broad-spectrum disease resistance from the associated costs on plant growth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Pieterse, C.M.J., Van der Does, D., Zamioudis, C., Leon-Reyes, A. & Van Wees, S.C.M. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 28, 489–521 (2012).
Conrath, U. et al. Priming: getting ready for battle. Mol. Plant Microbe Interact. 19, 1062–1071 (2006).
Pastor, V., Luna, E., Mauch-Mani, B., Ton, J. & Flors, V. Primed plants do not forget. Environ. Exp. Bot. 94, 46–56 (2013).
Kohler, A., Schwindling, S. & Conrath, U. Benzothiadiazole-induced priming for potentiated responses to pathogen infection, wounding, and infiltration of water into leaves requires the NPR1/NIM1 gene in Arabidopsis. Plant Physiol. 128, 1046–1056 (2002).
Jung, H.W., Tschaplinski, T.J., Wang, L., Glazebrook, J. & Greenberg, J.T. Priming in systemic plant immunity. Science 324, 89–91 (2009).
van Hulten, M., Pelser, M., van Loon, L., Pieterse, C.M.J. & Ton, J. Costs and benefits of priming for defense in Arabidopsis. Proc. Natl. Acad. Sci. USA 103, 5602–5607 (2006).
Walters, D. & Heil, M. Costs and trade-offs associated with induced resistance. Physiol. Mol. Plant Pathol. 71, 3–17 (2007).
Ahmad, S., Gordon-Weeks, R., Pickett, J. & Ton, J. Natural variation in priming of basal resistance: from evolutionary origin to agricultural exploitation. Mol. Plant Pathol. 11, 817–827 (2010).
Conrath, U. Molecular aspects of defence priming. Trends Plant Sci. 16, 524–531 (2011).
Luna, E., Bruce, T.J.A., Roberts, M.R., Flors, V. & Ton, J. Next-generation systemic acquired resistance. Plant Physiol. 158, 844–853 (2012).
Slaughter, A. et al. Descendants of primed Arabidopsis plants exhibit resistance to biotic stress. Plant Physiol. 158, 835–843 (2012).
Beckers, G.J.M. & Conrath, U. Priming for stress resistance: from the lab to the field. Curr. Opin. Plant Biol. 10, 425–431 (2007).
Gao, Q.M., Kachroo, A. & Kachroo, P. Chemical inducers of systemic immunity in plants. J. Exp. Bot. 10.1093/jxb/eru010 (2014).
Walters, D.R., Ratsep, J. & Havis, N.D. Controlling crop diseases using induced resistance: challenges for the future. J. Exp. Bot. 64, 1263–1280 (2013).
Cohen, Y.R. β-aminobutyric acid–induced resistance against plant pathogens. Plant Dis. 86, 448–457 (2002).
Zimmerli, L., Jakab, G., Métraux, J.-P. & Mauch-Mani, B. Potentiation of pathogen-specific defense mechanisms in Arabidopsis by β-aminobutyric acid. Proc. Natl. Acad. Sci. USA 97, 12920–12925 (2000).
Ton, J. et al. Dissecting the β-aminobutyric acid–induced priming phenomenon in Arabidopsis. Plant Cell 17, 987–999 (2005).
Singh, P. et al. The lectin receptor kinase-VI.2 is required for priming and positively regulates Arabidopsis pattern-triggered immunity. Plant Cell 24, 1256–1270 (2012).
Návarová, H., Bernsdorff, F., Döring, A.-C. & Zeier, J. Pipecolic acid, an endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity 24, 5123–5141 (2012).
Ton, J. & Mauch-Mani, B. β-amino-butyric acid–induced resistance against necrotrophic pathogens is based on ABA-dependent priming for callose. Plant J. 38, 119–130 (2004).
Van der Ent, S. et al. Priming of plant innate immunity by rhizobacteria and β-aminobutyric acid: differences and similarities in regulation. New Phytol. 183, 419–431 (2009).
Wu, C.-C., Singh, P., Chen, M.-C. & Zimmerli, L. L-Glutamine inhibits β-aminobutyric acid–induced stress resistance and priming in Arabidopsis. J. Exp. Bot. 61, 995–1002 (2010).
Delaney, T.P. et al. A central role of salicylic acid in plant disease resistance. Science 266, 1247–1250 (1994).
Flors, V. et al. Interplay between JA, SA and ABA signalling during basal and induced resistance against Pseudomonas syringae and Alternaria brassicicola. Plant J. 54, 81–92 (2008).
Parker, J.E. et al. The Arabidopsis downy mildew resistance gene RPP5 shares similarity to the toll and interleukin-1 receptors with N and L6. Plant Cell 9, 879–894 (1997).
Bell, C.J. & Ecker, J.R. Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics 19, 137–144 (1994).
Mauch-Mani, B. & Slusarenko, A.J. Production of salicylic acid precursors is a major function of phenylalanine ammonia-lyase in the resistance of Arabidopsis to Peronospora parasitica. Plant Cell 8, 203–212 (1996).
Guo, M., Yang, X.L. & Schimmel, P. New functions of aminoacyl-tRNA synthetases beyond translation. Nat. Rev. Mol. Cell Biol. 11, 668–674 (2010).
Nelson, B.K., Cai, X. & Nebenfuhr, A. A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 51, 1126–1136 (2007).
Duchêne, A.M. et al. Dual targeting is the rule for organellar aminoacyl-tRNA synthetases in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 102, 16484–16489 (2005).
Cohen, Y. 3-Aminobutyric acid induces systemic resistance against Peronospore tabacina. Physiol. Mol. Plant Pathol. 44, 273–288 (1994).
Silué, D., Pajot, E. & Cohen, Y. Induction of resistance to downy mildew (Peronospora parasitica) in cauliflower by DL-β-amino-n-butanoic acid (BABA). Plant Pathol. 51, 97–102 (2002).
Cohen, Y., Rubin, A.E. & Kilfin, G. Mechanisms of induced resistance in lettuce against Bremia lactucae by DL-β-amino-butyric acid (BABA). Eur. J. Plant Pathol. 126, 553–573 (2010).
Cavarelli, J. et al. The active site of yeast aspartyl-tRNA synthetase: structural and functional aspects of the aminoacylation reaction. EMBO J. 13, 327–337 (1994).
Schmitt, E. et al. Crystal structure of aspartyl-tRNA synthetase from Pyrococcus kodakaraensis KOD: archaeon specificity and catalytic mechanism of adenylate formation. EMBO J. 17, 5227–5237 (1998).
Shrift, A., Bechard, D. & Harcup, C. Utilization of selenocysteine by a cysteinyl-tRNA synthetase from Phaseolus aureus. Plant Physiol. 58, 248–252 (1976).
Ulmasov, B., Topin, A., Chen, Z., He, S.H. & Folk, W.R. Identity elements and aminoacylation of plant tRNATrp. Nucleic Acids Res. 26, 5139–5141 (1998).
Lloyd, A.J., Thomann, H.U., Ibba, M. & Soll, D. A broadly applicable continuous spectrophotometric assay for measuring aminoacyl-tRNA synthetase activity. Nucleic Acids Res. 23, 2886–2892 (1995).
Cestari, I. & Stuart, K. A spectrophotometric assay for quantitative measurement of aminoacyl-tRNA synthetase activity. J. Biomol. Screen. 18, 490–497 (2013).
Dever, T.E. & Hinnebusch, A.G. GCN2 whets the appetite for amino acids. Mol. Cell 18, 141–142 (2005).
Li, M.W., AuYeung, W.K. & Lam, H.M. The GCN2 homologue in Arabidopsis thaliana interacts with uncharged tRNA and uses Arabidopsis eIF2α molecules as direct substrates. Plant Biol. 15, 13–18 (2013).
Zhang, Y. et al. GCN2-dependent phosphorylation of eukaryotic translation initiation factor-2α in Arabidopsis. J. Exp. Bot. 59, 3131–3141 (2008).
Holub, E.B., Beynon, L.J. & Crute, I.R. Phenotypic and genotypic characterization of interactions between isolates of Peronospora parasitica and accessions of Arabidopsis thaliana. Mol. Plant Microbe Interact. 7, 223–239 (1994).
Guo, M. & Schimmel, P. Essential nontranslational functions of tRNA synthetases. Nat. Chem. Biol. 9, 145–153 (2013).
Ko, Y.-G. et al. Glutamine-dependent antiapoptotic interaction of human glutaminyl-tRNA synthetase with apoptosis signal-regulating kinase 1. J. Biol. Chem. 276, 6030–6036 (2001).
Frye, C.A., Tang, D. & Innes, R.W. Negative regulation of defense responses in plants by a conserved MAPKK kinase. Proc. Natl. Acad. Sci. USA 98, 373–378 (2001).
Kong, Q. et al. The MEKK1–MKK1/MKK2–MPK4 kinase cascade negatively regulates immunity mediated by a mitogen-activated protein kinase kinase kinase in Arabidopsis. Plant Cell 24, 2225–2236 (2012).
Guo, M., Yang, X.L. & Schimmel, P. New functions of aminoacyl-tRNA synthetases beyond translation. Nat. Rev. Mol. Cell Biol. 11, 668–674 (2010).
Kim, Y., Schumaker, K. & Zhu, J.-K. in Arabidopsis Protocols Vol. 323 (eds. Salinas, J. & Sanchez-Serrano, J.) 101–103 (Humana Press, 2006).
Audenaert, K., De Meyer, G.B. & Hofte, M.M. Abscisic acid determines basal susceptibility of tomato to Botrytis cinerea and suppresses salicylic acid–dependent signaling mechanisms. Plant Physiol. 128, 491–501 (2002).
Earley, K.W. et al. Gateway-compatible vectors for plant functional genomics and proteomics. Plant J. 45, 616–629 (2006).
Lin, J.J. Optimization of the transformation efficiency of Agrobacterium tumefaciens cells using electroporation. Plant Sci. 101, 11–15 (1994).
Clough, S.J. & Bent, A.F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Berkowitz, O., Jost, R., Pollmann, S. & Masle, J. Characterization of TCTP, the translationally controlled tumor protein, from Arabidopsis thaliana. Plant Cell 20, 3430–3447 (2008).
Pétriacq, P. et al. Inducible NAD overproduction in Arabidopsis alters metabolic pools and gene expression correlated with increased salicylate content and resistance to Pst-AvrRpm1. Plant J. 70, 650–665 (2012).
Czechowski, T., Stitt, M., Altmann, T., Udvardi, M.K. & Scheible, W.R. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 139, 5–17 (2005).
Accelrys Software Inc., Discovery Studio Modeling Environment, Release 3.5 (San Diego, Accelrys Software Inc., 2012).
Wu, G., Robertson, D.H., Brooks, C.L. & Vieth, M. Detailed analysis of grid-based molecular docking: a case study of CDOCKER—A CHARMm-based MD docking algorithm. J. Comput. Chem. 24, 1549–1562 (2003).
Acknowledgements
We thank S. van Wees, N. Halford, J. Lucas, J. Pickett, I. Feussner, X. Zhang, W. Kegge, D. Acoska, M. Roberts, V. Pastor, J. Gamir and B. Mauch-Mani for fruitful discussions, practical assistance and/or helpful feedback. Arabidopsis NahG B15 and Ler rpp5 seeds were kindly provided by J. Ryals (Research Triangle Park, USA) and J. Parker (Max Planck Institute, Cologne), respectively. The research was supported by a VENI grant to J.T. (no. 863.04.019) from the Netherlands Organisation of Scientific Research (NWO), a Biotechnology and Biological Sciences Research Council Institute Career Path Fellowship (no. BB/E023959/1) to J.T., a consolidator grant from the European Research Council (no. 309944-Prime-A-Plant) to J.T., a Research Leadership Award from the Leverhulme Trust (no. RL-2012-042) to J.T., European Union Seventh Framework Programme (FP7/2007-2013; n°265865-PURE) to J.T., a grant from the Felix Thornley Cobbold Agricultural Trust to J.T. and E.L. and a VICI grant (no. 865.04.002) to C.M.J.P. from NWO.
Author information
Authors and Affiliations
Contributions
J.T. designed and supervised the research plan, performed experiments (mutant screen, map-based cloning, construction of transgenic lines, RT-qPCR and bioassays) and wrote the manuscript. E.L. performed experiments (map-based cloning, construction of transgenic lines, RT-qPCR, bioassays, confocal microscopy and IP assays), wrote the manuscript and provided intellectual input. M.v.H. performed experiments (mutant screen and bioassays) and provided intellectual input. Y.Z. performed experiments (map-based cloning and RT-qPCR) and provided intellectual input. O.B. performed experiments (GCN2 activity assays) and provided intellectual input. A.L. performed experiments (confocal microscopy and IP assays). P.P. performed experiments (MS). M.A.S. did computational modeling. B.C. did computational modeling and provided intellectual input. M.B. provided intellectual input. A.v.d.M. performed experiments (confocal microscopy). C.M.J.P. provided intellectual input. V.F. performed experiments (bioassays) and provided intellectual input.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Figures 1–16 and Supplementary Tables 1 and 2. (PDF 17925 kb)
Rights and permissions
About this article
Cite this article
Luna, E., van Hulten, M., Zhang, Y. et al. Plant perception of β-aminobutyric acid is mediated by an aspartyl-tRNA synthetase. Nat Chem Biol 10, 450–456 (2014). https://doi.org/10.1038/nchembio.1520
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1520
This article is cited by
-
Aspartyl tRNA-synthetase (AspRS) gene family enhances drought tolerance in poplar through BABA-PtrIBIs-PtrVOZ signaling module
BMC Genomics (2023)
-
Simultaneous determination of all aminobutyric acids by chiral derivatization and liquid chromatography-tandem mass spectrometry
Analytical Sciences (2023)
-
Genome-wide association mapping for identification of sheath blight resistance loci from wild rice Oryza rufipogon
Euphytica (2022)
-
The synergistic benefits of β-aminobutyric acid and γ-aminobutyrate on salt and drought tolerance in cassava
Plant Biotechnology Reports (2022)
-
Disclosure of salicylic acid and jasmonic acid-responsive genes provides a molecular tool for deciphering stress responses in soybean
Scientific Reports (2021)