Abstract
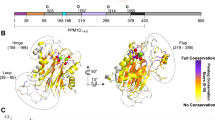
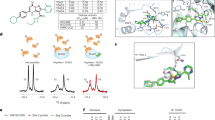
Although therapeutic interventions of signal-transduction cascades with targeted kinase inhibitors are a well-established strategy, drug-discovery efforts to identify targeted phosphatase inhibitors have proven challenging. Herein we report a series of allosteric, small-molecule inhibitors of wild-type p53-induced phosphatase (Wip1), an oncogenic phosphatase common to multiple cancers. Compound binding to Wip1 is dependent on a 'flap' subdomain located near the Wip1 catalytic site that renders Wip1 structurally divergent from other members of the protein phosphatase 2C (PP2C) family and that thereby confers selectivity for Wip1 over other phosphatases. Treatment of tumor cells with the inhibitor GSK2830371 increases phosphorylation of Wip1 substrates and causes growth inhibition in both hematopoietic tumor cell lines and Wip1-amplified breast tumor cells harboring wild-type TP53. Oral administration of Wip1 inhibitors in mice results in expected pharmacodynamic effects and causes inhibition of lymphoma xenograft growth. To our knowledge, GSK2830371 is the first orally active, allosteric inhibitor of Wip1 phosphatase.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Lu, X. et al. The type 2C phosphatase Wip1: an oncogenic regulator of tumor suppressor and DNA damage response pathways. Cancer Metastasis Rev. 27, 123–135 (2008).
Lowe, J. et al. Regulation of the Wip1 phosphatase and its effects on the stress response. Front. Biosci. 17, 1480–1498 (2012).
Zhu, Y.H. & Bulavin, D.V. Wip1-Dependent Signaling Pathways in Health and Diseases in Progress in Molecular Biology and Translational Science Protein Phosphorylation in Health and Disease (ed. Shirish, S.) 307–325 (Academic Press, 2012).
Fiscella, M. et al. Wip1, a novel human protein phosphatase that is induced in response to ionizing radiation in a p53-dependent manner. Proc. Natl. Acad. Sci. USA 94, 6048–6053 (1997).
Yamaguchi, H. et al. Substrate specificity of the human protein phosphatase 2Cδ, Wip1. Biochemistry 44, 5285–5294 (2005).
Yamaguchi, H., Durell, S.R., Chatterjee, D.K., Anderson, C.W. & Appella, E. The Wip1 phosphatase PPM1D dephosphorylates SQ/TQ motifs in checkpoint substrates phosphorylated by PI3K-like kinases. Biochemistry 46, 12594–12603 (2007).
Schito, M.L., Demidov, O.N., Saito, S., Ashwell, J.D. & Appella, E. Wip1 phosphatase-deficient mice exhibit defective T cell maturation due to sustained p53 activation. J. Immunol. 176, 4818–4825 (2006).
Choi, J. et al. Mice deficient for the wild-type p53-induced phosphatase gene (Wipl) exhibit defects in reproductive organs, immune function, and cell cycle control. Mol. Cell Biol. 22, 1094–1105 (2002).
Castellino, R.C. et al. Medulloblastomas overexpress the p53-inactivating oncogene WIP1/PPM1D. J. Neurooncol. 86, 245–256 (2008).
Fuku, T., Semba, S., Yutori, H. & Yokozaki, H. Increased wild-type p53-induced phosphatase 1 (Wip1 or PPM1D) expression correlated with downregulation of checkpoint kinase 2 in human gastric carcinoma. Pathol. Int. 57, 566–571 (2007).
Hirasawa, A. et al. Association of 17q21-q24 gain in ovarian clear cell adenocarcinomas with poor prognosis and identification of PPM1D and APPBP2 as likely amplification targets. Clin. Cancer Res. 9, 1995–2004 (2003).
Li, J. et al. Oncogenic properties of PPM1D located within a breast cancer amplification epicenter at 17q23. Nat. Genet. 31, 133–134 (2002).
Hu, X. et al. Genetic alterations and oncogenic pathways associated with breast cancer subtypes. Mol. Cancer Res. 7, 511–522 (2009).
Rauta, J. et al. The serine-threonine protein phosphatase PPM1D is frequently activated through amplification in aggressive primary breast tumours. Breast Cancer Res. Treat. 95, 257–263 (2006).
Natrajan, R. et al. Tiling path genomic profiling of grade 3 invasive ductal breast cancers. Clin. Cancer Res. 15, 2711–2722 (2009).
Saito-Ohara, F. et al. PPM1D is a potential target for 17q gain in neuroblastoma. Cancer Res. 63, 1876–1883 (2003).
Tan, D.S.P. et al. PPM1D is a potential therapeutic target in ovarian clear cell carcinomas. Clin. Cancer Res. 15, 2269–2280 (2009).
Bulavin, D.V. et al. Amplification of PPM1D in human tumors abrogates p53 tumor-suppressor activity. Nat. Genet. 31, 210–215 (2002).
Demidov, O.N. et al. The role of the MKK6/p38 MAPK pathway in Wip1-dependent regulation of ErbB2-driven mammary gland tumorigenesis. Oncogene 26, 2502–2506 (2007).
Lambros, M.B. et al. PPM1D gene amplification and overexpression in breast cancer: A qRT-PCR and chromogenic in situ hybridization study. Mod. Pathol. 23, 1334–1345 (2010).
Satoh, N. et al. Oncogenic phosphatase Wip1 is a novel prognostic marker for lung adenocarcinoma patient survival. Cancer Sci. 102, 1101–1106 (2011).
Vintonyak, V.V., Antonchick, A.P., Rauh, D. & Waldmann, H. The therapeutic potential of phosphatase inhibitors. Curr. Opin. Chem. Biol. 13, 272–283 (2009).
Clark, M.A. et al. Design, synthesis and selection of DNA-encoded small-molecule libraries. Nat. Chem. Biol. 5, 647–654 (2009).
Yamaguchi, H. et al. Development of a substrate-based cyclic phosphopeptide inhibitor of protein phosphatase 2Cδ, Wip1. Biochemistry 45, 13193–13202 (2006).
Chuman, Y. et al. Characterization of the active site and a unique uncompetitive inhibitor of the PPM1-type protein phosphatase PPM1D. Protein Pept. Lett. 15, 938–948 (2008).
Pullen, K.E. et al. An alternate conformation and a third metal in PstP/Ppp, the M. tuberculosis PP2C-family Ser/Thr protein phosphatase. Structure 12, 1947–1954 (2004).
Schlicker, C. et al. Structural analysis of the PP2C phosphatase tPphA from Thermosynechococcus elongatus: a flexible flap subdomain controls access to the catalytic site. J. Mol. Biol. 376, 570–581 (2008).
Rayter, S. et al. A chemical inhibitor of PPM1D that selectively kills cells overexpressing PPM1D. Oncogene 27, 1036–1044 (2008).
Kim, W. et al. Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol. Cell 44, 325–340 (2011).
Wagner, S.A. et al. A proteome-wide, quantitative survey of in vivo ubiquitylation sites reveals widespread regulatory roles. Mol. Cell Proteomics 10, M111.013284 (2011).
Ong, S.E. The expanding field of SILAC. Anal. Bioanal. Chem. 404, 967–976 (2012).
Hayashi, R. et al. Optimization of a cyclic peptide inhibitor of Ser/Thr phosphatase PPM1D (Wip1). Biochemistry 50, 4537–4549 (2011).
Tanoue, K. et al. Binding of a third metal ion by the human phosphatases PP2Cα and Wip1 is required for phosphatase activity. Biochemistry 52, 5830–5843 (2013).
Acknowledgements
We thank M. Sarpong, C. Donatelli, J. Deng, T. Graybill, K.O. Johanson, T. Rust, T. Jurewicz and T. Tomaszek for their contributions in the identification and characterization of this compound series.
Author information
Authors and Affiliations
Contributions
A.G.G., M.R., A.G., S.-Y.Z., E.M. and R.K. conducted cellular and in vivo studies; T.H.F., M.A.S., M.G.D., X.P. and D.A.H. conducted medicinal chemistry; K.F., J.Y., B.P., E.D., A.J. and M.B. conducted enzymatic assays and analysis; J.-P.J., M.S. and S.M. conducted the HTS and thermal shift assays; D.E.M. conducted proteomic analysis; R.H.S., H.Z., R.B.K. and B.S. conducted gene expression and protein purification; and N.N. and G.C. conducted computational analysis and protein construct design. A.G.G., M.R., T.H.F., D.A.H. and R.K. wrote the manuscript, which was reviewed by all authors.
Corresponding authors
Ethics declarations
Competing interests
All authors are employees of GlaxoSmithKline and several have stock ownership of GlaxoSmithKline.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Figures 1-22, Supplementary Tables 1-4 and Supplementary Note. (PDF 5930 kb)
Rights and permissions
About this article
Cite this article
Gilmartin, A., Faitg, T., Richter, M. et al. Allosteric Wip1 phosphatase inhibition through flap-subdomain interaction. Nat Chem Biol 10, 181–187 (2014). https://doi.org/10.1038/nchembio.1427
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1427
This article is cited by
-
Wip1 inhibits neutrophil extracellular traps to promote abscess formation in mice by directly dephosphorylating Coronin-1a
Cellular & Molecular Immunology (2023)
-
Targeting protein phosphatases in cancer immunotherapy and autoimmune disorders
Nature Reviews Drug Discovery (2023)
-
Co-targeting WIP1 and PARP induces synthetic lethality in hepatocellular carcinoma
Cell Communication and Signaling (2022)
-
DNA-encoded chemical libraries
Nature Reviews Methods Primers (2022)
-
PPM1D suppresses p53-dependent transactivation and cell death by inhibiting the Integrated Stress Response
Nature Communications (2022)