Abstract
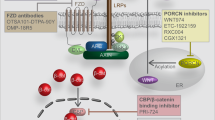
The pervasive influence of secreted Wnt signaling proteins in tissue homeostasis and tumorigenesis has galvanized efforts to identify small molecules that target Wnt-mediated cellular responses. By screening a diverse synthetic chemical library, we have discovered two new classes of small molecules that disrupt Wnt pathway responses; whereas one class inhibits the activity of Porcupine, a membrane-bound acyltransferase that is essential to the production of Wnt proteins, the other abrogates destruction of Axin proteins, which are suppressors of Wnt/β-catenin pathway activity. With these small molecules, we establish a chemical genetic approach for studying Wnt pathway responses and stem cell function in adult tissue. We achieve transient, reversible suppression of Wnt/β-catenin pathway response in vivo, and we establish a mechanism-based approach to target cancerous cell growth. The signal transduction mechanisms shown here to be chemically tractable additionally contribute to Wnt-independent signal transduction pathways and thus could be broadly exploited for chemical genetics and therapeutic goals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Clevers, H. Wnt/beta-catenin signaling in development and disease. Cell 127, 469–480 (2006).
Cole, M.F., Johnstone, S.E., Newman, J.J., Kagey, M.H. & Young, R.A. Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev. 22, 746–755 (2008).
Van der Flier, L.G. et al. The intestinal Wnt/TCF signature. Gastroenterology 132, 628–632 (2007).
Fevr, T., Robine, S., Louvard, D. & Huelsken, J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol. Cell. Biol. 27, 7551–7559 (2007).
Korinek, V. et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 19, 379–383 (1998).
Muncan, V. et al. T-cell factor 4 (Tcf7l2) maintains proliferative compartments in zebrafish intestine. EMBO Rep. 8, 966–973 (2007).
Brack, A.S. et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317, 807–810 (2007).
Liu, H. et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science 317, 803–806 (2007).
Reya, T. & Clevers, H. Wnt signalling in stem cells and cancer. Nature 434, 843–850 (2005).
Kinzler, K.W. & Vogelstein, B. Lessons from hereditary colorectal cancer. Cell 87, 159–170 (1996).
Sjoblom, T. et al. The consensus coding sequences of human breast and colorectal cancers. Science 314, 268–274 (2006).
Polakis, P. The many ways of Wnt in cancer. Curr. Opin. Genet. Dev. 17, 45–51 (2007).
Takada, R. et al. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev. Cell 11, 791–801 (2006).
Kurayoshi, M., Yamamoto, H., Izumi, S. & Kikuchi, A. Post-translational palmitoylation and glycosylation of Wnt-5a are necessary for its signalling. Biochem. J. 402, 515–523 (2007).
Chamoun, Z. et al. Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science 293, 2080–2084 (2001).
Abrami, L., Kunz, B., Iacovache, I. & van der Goot, F.G. Palmitoylation and ubiquitination regulate exit of the Wnt signaling protein LRP6 from the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 105, 5384–5389 (2008).
Tanaka, K., Okabayashi, K., Asashima, M., Perrimon, N. & Kadowaki, T. The evolutionarily conserved porcupine gene family is involved in the processing of the Wnt family. Eur. J. Biochem. 267, 4300–4311 (2000).
Huang, H. & He, X. Wnt/beta-catenin signaling: new (and old) players and new insights. Curr. Opin. Cell Biol. 20, 119–125 (2008).
Orsulic, S., Huber, O., Aberle, H., Arnold, S. & Kemler, R. E-cadherin binding prevents beta-catenin nuclear localization and beta-catenin/LEF-1-mediated transactivation. J. Cell Sci. 112, 1237–1245 (1999).
Jho, E.H. et al. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell. Biol. 22, 1172–1183 (2002).
Lustig, B. et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol. Cell. Biol. 22, 1184–1193 (2002).
Cselenyi, C.S. et al. LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3's phosphorylation of beta-catenin. Proc. Natl. Acad. Sci. USA 105, 8032–8037 (2008).
Stoick-Cooper, C.L. et al. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development 134, 479–489 (2007).
Whitehead, G.G., Makino, S., Lien, C.L. & Keating, M.T. fgf20 is essential for initiating zebrafish fin regeneration. Science 310, 1957–1960 (2005).
Sabates-Bellver, J. et al. Transcriptome profile of human colorectal adenomas. Mol. Cancer Res. 5, 1263–1275 (2007).
Barker, N. & Clevers, H. Mining the Wnt pathway for cancer therapeutics. Nat. Rev. Drug Discov. 5, 997–1014 (2006).
Yang, J., Brown, M.S., Liang, G., Grishin, N.V. & Goldstein, J.L. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 132, 387–396 (2008).
Lee, E., Salic, A., Kruger, R., Heinrich, R. & Kirschner, M.W. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 1, E10 (2003).
Shepard, J.L. et al. A zebrafish bmyb mutation causes genome instability and increased cancer susceptibility. Proc. Natl. Acad. Sci. USA 102, 13194–13199 (2005).
Poss, K.D. et al. Roles for Fgf signaling during zebrafish fin regeneration. Dev. Biol. 222, 347–358 (2000).
Acknowledgements
We thank M. Bienz (Medical Research Council Laboratory of Molecular Biology), R.T. Moon (University of Washington), P.T. Chuang (University of California, San Francisco), J. Laborda (University of Castilla-La Mancha), G. Johnson (University of Alabama at Birmingham), P. Beachy (Stanford University), J. Minna, M. Brown and J. Goldstein (University of Texas Southwestern Medical Center) for reagents, and D. Frantz, K. Lillard, the University of Texas Southwestern Pathology Core, S. McKnight and the High-Throughput Screening Core for support with the chemical screen. This work was supported by the US National Cancer Institute (PO1 CA095471; Z.M., J.K. and N.S.W.), the US National Institute of General Medical Sciences (1R01GM076398-01), the American Cancer Society (RSG GMC-112251), the Welch Foundation (I-1665), a High Risk/High Impact award from the University of Texas Southwestern and an endowment from Virginia Murchison Linthicum.
Author information
Authors and Affiliations
Contributions
B.C., M.E.D., W.T., C.-W.F., S.W., W.H., J.K., N.S.W., M.G.R., J.F.A., C.C. and L.L. designed the experiments and analyzed the results. B.C., M.E.D., C.C. and L.L. wrote the manuscript. J.F.A. and B.C. performed zebrafish experiments. J.L., Z.M. and C.C. synthesized compounds.
Corresponding author
Ethics declarations
Competing interests
The authors declare competing financial interests in the form of a pending patent application.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Methods (PDF 2574 kb)
Rights and permissions
About this article
Cite this article
Chen, B., Dodge, M., Tang, W. et al. Small molecule–mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol 5, 100–107 (2009). https://doi.org/10.1038/nchembio.137
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.137
This article is cited by
-
WNT signalling control by KDM5C during development affects cognition
Nature (2024)
-
Interactions of melatonin with various signaling pathways: implications for cancer therapy
Cancer Cell International (2022)
-
Ninjurin1 drives lung tumor formation and progression by potentiating Wnt/β-Catenin signaling through Frizzled2-LRP6 assembly
Journal of Experimental & Clinical Cancer Research (2022)
-
Chemical genomics with pyrvinium identifies C1orf115 as a regulator of drug efflux
Nature Chemical Biology (2022)
-
CUGBP1, a crucial factor for heart regeneration in mice
Cell Death & Disease (2022)