Abstract
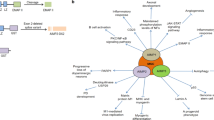
Nontranslational functions of vertebrate aminoacyl tRNA synthetases (aaRSs), which catalyze the production of aminoacyl-tRNAs for protein synthesis, have recently been discovered. Although these new functions were thought to be 'moonlighting activities', many are as critical for cellular homeostasis as their activity in translation. New roles have been associated with their cytoplasmic forms as well as with nuclear and secreted extracellular forms that affect pathways for cardiovascular development and the immune response and mTOR, IFN-γ and p53 signaling. The associations of aaRSs with autoimmune disorders, cancers and neurological disorders further highlight nontranslational functions of these proteins. New architecture elaborations of the aaRSs accompany their functional expansion in higher organisms and have been associated with the nontranslational functions for several aaRSs. Although a general understanding of how these functions developed is limited, the expropriation of aaRSs for essential nontranslational functions may have been initiated by co-opting the amino acid–binding site for another purpose.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
22 February 2012
In the version of this article initially published online, Min Guo's name was misspelled. The error has been corrected in the HTML version of the article.
References
Carter, C.W. Jr. Cognition, mechanism, and evolutionary relationships in aminoacyl-tRNA synthetases. Annu. Rev. Biochem. 62, 715–748 (1993).
Ibba, M. & Söll, D. Aminoacyl-tRNA synthesis. Annu. Rev. Biochem. 69, 617–650 (2000).
Ryckelynck, M., Giegé, R. & Frugier, M. tRNAs and tRNA mimics as cornerstones of aminoacyl-tRNA synthetase regulations. Biochimie 87, 835–845 (2005).
Putney, S.D. & Schimmel, P. An aminoacyl tRNA synthetase binds to a specific DNA sequence and regulates its gene transcription. Nature 291, 632–635 (1981).
Sarkar, J., Poruri, K., Boniecki, M.T., McTavish, K.K. & Martinis, S.A. Yeast mitochondrial leucyl-tRNA synthetase CP1 domain has functionally diverged to accommodate RNA splicing at expense of hydrolytic editing. J. Biol. Chem. 287, 14772–14781 (2012).
Paukstelis, P.J., Chen, J.H., Chase, E., Lambowitz, A.M. & Golden, B.L. Structure of a tyrosyl-tRNA synthetase splicing factor bound to a group I intron RNA. Nature 451, 94–97 (2008).
Arnez, J.G. & Moras, D. Structural and functional considerations of the aminoacylation reaction. Trends Biochem. Sci. 22, 211–216 (1997).
Torres-Larios, A., Sankaranarayanan, R., Rees, B., Dock-Bregeon, A.C. & Moras, D. Conformational movements and cooperativity upon amino acid, ATP and tRNA binding in threonyl-tRNA synthetase. J. Mol. Biol. 331, 201–211 (2003).
Jain, M. et al. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science 336, 1040–1044 (2012).
Possemato, R. et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 476, 346–350 (2011).
Wang, J. et al. Dependence of mouse embryonic stem cells on threonine catabolism. Science 325, 435–439 (2009).
Kim, J. & Guan, K.L. Amino acid signaling in TOR activation. Annu. Rev. Biochem. 80, 1001–1032 (2011).
Han, J.M. et al. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell 149, 410–424 (2012).
Segev, N. & Hay, N. Hijacking leucyl-tRNA synthetase for amino acid-dependent regulation of TORC1. Mol. Cell 46, 4–6 (2012).
Hoffman, R.M. Tumor-seeking Salmonella amino acid auxotrophs. Curr. Opin. Biotechnol. 22, 917–923 (2011).
Ruggiero, R.A. et al. Concomitant tumor resistance: the role of tyrosine isomers in the mechanisms of metastases control. Cancer Res. 72, 1043–1050 (2012).
Levine, A.J. & Puzio-Kuter, A.M. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science 330, 1340–1344 (2010).
DeBerardinis, R.J. & Cheng, T. Q's next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 29, 313–324 (2010).
Wise, D.R. et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. USA 105, 18782–18787 (2008).
Hattori, K., Naguro, I., Runchel, C. & Ichijo, H. The roles of ASK family proteins in stress responses and diseases. Cell Commun. Signal. 7, 9 (2009).
Ko, Y.G. et al. Glutamine-dependent antiapoptotic interaction of human glutaminyl-tRNA synthetase with apoptosis signal-regulating kinase 1. J. Biol. Chem. 276, 6030–6036 (2001).
Wahab, S.Z. & Yang, D.C. Synthesis of diadenosine 5′,5′′′ -P1,P4-tetraphosphate by lysyl-tRNA synthetase and a multienzyme complex of aminoacyl-tRNA synthetases from rat liver. J. Biol. Chem. 260, 5286–5289 (1985).
Zamecnik, P. Diadenosine 5′,5′′′-P1,P4-tetraphosphate (Ap4A): its role in cellular metabolism. Anal. Biochem. 134, 1–10 (1983).
Bochner, B.R., Lee, P.C., Wilson, S.W., Cutler, C.W. & Ames, B.N. AppppA and related adenylylated nucleotides are synthesized as a consequence of oxidation stress. Cell 37, 225–232 (1984).
Lee, Y.N., Nechushtan, H., Figov, N. & Razin, E. The function of lysyl-tRNA synthetase and Ap4A as signaling regulators of MITF activity in FcδRI-activated mast cells. Immunity 20, 145–151 (2004).
Yannay-Cohen, N. et al. LysRS serves as a key signaling molecule in the immune response by regulating gene expression. Mol. Cell 34, 603–611 (2009).
Ofir-Birin, Y. et al. Structural switch of lysyl-tRNA synthetases between translation and transcription. Mol. Cell 49, 30–42 (2013).
Blanquet, S., Plateau, P. & Brevet, A. The role of zinc in 5′,5′-diadenosine tetraphosphate production by aminoacyl-transfer RNA synthetases. Mol. Cell. Biochem. 52, 3–11 (1983).
Justin, N., De Marco, V., Aasland, R. & Gamblin, S.J. Reading, writing and editing methylated lysines on histone tails: new insights from recent structural studies. Curr. Opin. Struct. Biol. 20, 730–738 (2010).
Tzima, E. et al. VE-cadherin links tRNA synthetase cytokine to anti-angiogenic function. J. Biol. Chem. 280, 2405–2408 (2005).
Guo, M., Yang, X.L. & Schimmel, P. New functions of aminoacyl-tRNA synthetases beyond translation. Nat. Rev. Mol. Cell Biol. 11, 668–674 (2010).
Shiba, K. Intron positions delineate the evolutionary path of a pervasively appended peptide in five human aminoacyl-tRNA synthetases. J. Mol. Evol. 55, 727–733 (2002).
Wakasugi, K. et al. A human aminoacyl-tRNA synthetase as a regulator of angiogenesis. Proc. Natl. Acad. Sci. USA 99, 173–177 (2002).
Sajish, M. et al. Trp-tRNA synthetase bridges DNA-PKcs to PARP-1 to link IFN-γ and p53 signaling. Nat. Chem. Biol. 8, 547–554 (2012).
Ray, P.S. et al. Evolution of function of a fused metazoan tRNA synthetase. Mol. Biol. Evol. 28, 437–447 (2011).
Cerini, C. et al. A component of the multisynthetase complex is a multifunctional aminoacyl-tRNA synthetase. EMBO J. 10, 4267–4277 (1991).
Arif, A., Jia, J., Moodt, R.A., DiCorleto, P.E. & Fox, P.L. Phosphorylation of glutamyl-prolyl tRNA synthetase by cyclin-dependent kinase 5 dictates transcript-selective translational control. Proc. Natl. Acad. Sci. USA 108, 1415–1420 (2011).
Mukhopadhyay, R., Jia, J., Arif, A., Ray, P.S. & Fox, P.L. The GAIT system: a gatekeeper of inflammatory gene expression. Trends Biochem. Sci. 34, 324–331 (2009).
Jia, J., Arif, A., Ray, P.S. & Fox, P.L. WHEP domains direct noncanonical function of glutamyl-prolyl tRNA synthetase in translational control of gene expression. Mol. Cell 29, 679–690 (2008).
Herzog, W., Muller, K., Huisken, J. & Stainier, D.Y. Genetic evidence for a noncanonical function of seryl-tRNA synthetase in vascular development. Circ. Res. 104, 1260–1266 (2009).
Fukui, H., Hanaoka, R. & Kawahara, A. Noncanonical activity of seryl-tRNA synthetase is involved in vascular development. Circ. Res. 104, 1253–1259 (2009).
Xu, X. et al. Unique domain appended to vertebrate tRNA synthetase is essential for vascular development. Nat. Commun. 3, 681 (2012).
Francin, M., Kaminska, M., Kerjan, P. & Mirande, M. The N-terminal domain of mammalian lysyl-tRNA synthetase is a functional tRNA-binding domain. J. Biol. Chem. 277, 1762–1769 (2002).
Frugier, M., Moulinier, L. & Giegé, R. A domain in the N-terminal extension of class IIb eukaryotic aminoacyl-tRNA synthetases is important for tRNA binding. EMBO J. 19, 2371–2380 (2000).
Negrutskii, B.S., Shalak, V.F., Kerjan, P., El'fskaya, A.V. & Mirande, M. Functional interaction of mammalian valyl-tRNA synthetase with elongation factor EF-1α in the complex with EF-1H. J. Biol. Chem. 274, 4545–4550 (1999).
He, R., Zu, L.D., Yao, P., Chen, X. & Wang, E.D. Two non-redundant fragments in the N-terminal peptide of human cytosolic methionyl-tRNA synthetase were indispensable for the multi-synthetase complex incorporation and enzyme activity. Biochim. Biophys. Acta 1794, 347–354 (2009).
Kerjan, P., Cerini, C., Semeriva, M. & Mirande, M. The multienzyme complex containing nine aminoacyl-tRNA synthetases is ubiquitous from Drosophila to mammals. Biochim. Biophys. Acta 1199, 293–297 (1994).
Lee, S.W., Cho, B.H., Park, S.G. & Kim, S. Aminoacyl-tRNA synthetase complexes: beyond translation. J. Cell Sci. 117, 3725–3734 (2004).
Robinson, J.C., Kerjan, P. & Mirande, M. Macromolecular assemblage of aminoacyl-tRNA synthetases: quantitative analysis of protein-protein interactions and mechanism of complex assembly. J. Mol. Biol. 304, 983–994 (2000).
Quevillon, S., Robinson, J.C., Berthonneau, E., Siatecka, M. & Mirande, M. Macromolecular assemblage of aminoacyl-tRNA synthetases: identification of protein-protein interactions and characterization of a core protein. J. Mol. Biol. 285, 183–195 (1999).
Han, J.M. et al. Hierarchical network between the components of the multi-tRNA synthetase complex: implications for complex formation. J. Biol. Chem. 281, 38663–38667 (2006).
Kyriacou, S.V. & Deutscher, M.P. An important role for the multienzyme aminoacyl-tRNA synthetase complex in mammalian translation and cell growth. Mol. Cell 29, 419–427 (2008).
Kang, T. et al. AIMP3/p18 controls translational initiation by mediating the delivery of charged initiator tRNA to initiation complex. J. Mol. Biol. 423, 475–481 (2012).
Park, S.G., Choi, E.C. & Kim, S. Aminoacyl-tRNA synthetase-interacting multifunctional proteins (AIMPs): a triad for cellular homeostasis. IUBMB Life 62, 296–302 (2010).
Zhu, X. et al. MSC p43 required for axonal development in motor neurons. Proc. Natl. Acad. Sci. USA 106, 15944–15949 (2009).
Park, S.G. et al. The novel cytokine p43 stimulates dermal fibroblast proliferation and wound repair. Am. J. Pathol. 166, 387–398 (2005).
Kim, J.Y. et al. p38 is essential for the assembly and stability of macromolecular tRNA synthetase complex: implications for its physiological significance. Proc. Natl. Acad. Sci. USA 99, 7912–7916 (2002).
Kim, M.J. et al. Downregulation of FUSE-binding protein and c-myc by tRNA synthetase cofactor p38 is required for lung cell differentiation. Nat. Genet. 34, 330–336 (2003).
Choi, J.W., Um, J.Y., Kundu, J.K., Surh, Y.J. & Kim, S. Multidirectional tumor-suppressive activity of AIMP2/p38 and the enhanced susceptibility of AIMP2 heterozygous mice to carcinogenesis. Carcinogenesis 30, 1638–1644 (2009).
Ko, H.S. et al. Accumulation of the authentic parkin substrate aminoacyl-tRNA synthetase cofactor, p38/JTV-1, leads to catecholaminergic cell death. J. Neurosci. 25, 7968–7978 (2005).
Kwon, N.H. et al. Dual role of methionyl-tRNA synthetase in the regulation of translation and tumor suppressor activity of aminoacyl-tRNA synthetase-interacting multifunctional protein-3. Proc. Natl. Acad. Sci. USA 108, 19635–19640 (2011).
Park, B.J. et al. The haploinsufficient tumor suppressor p18 upregulates p53 via interactions with ATM/ATR. Cell 120, 209–221 (2005).
Kim, K.J. et al. Determination of three-dimensional structure and residues of the novel tumor suppressor AIMP3/p18 required for the interaction with ATM. J. Biol. Chem. 283, 14032–14040 (2008).
Howard, O.M. et al. Histidyl-tRNA synthetase and asparaginyl-tRNA synthetase, autoantigens in myositis, activate chemokine receptors on T lymphocytes and immature dendritic cells. J. Exp. Med. 196, 781–791 (2002).
Yamasaki, Y. et al. Unusually high frequency of autoantibodies to PL-7 associated with milder muscle disease in Japanese patients with polymyositis/dermatomyositis. Arthritis Rheum. 54, 2004–2009 (2006).
Xu, Z. et al. Internally deleted human tRNA synthetase suggests evolutionary pressure for repurposing. Structure 20, 1470–1477 (2012).
Wu, P.C. et al. In vivo sensitivity of human melanoma to tumor necrosis factor (TNF)-α is determined by tumor production of the novel cytokine endothelial-monocyte activating polypeptide II (EMAPII). Cancer Res. 59, 205–212 (1999).
Shalak, V. et al. The EMAPII cytokine is released from the mammalian multisynthetase complex after cleavage of its p43/proEMAPII component. J. Biol. Chem. 276, 23769–23776 (2001).
Zhou, Q. et al. Orthogonal use of a human tRNA synthetase active site to achieve multifunctionality. Nat. Struct. Mol. Biol. 17, 57–61 (2010).
Banin, E. et al. T2-TrpRS inhibits preretinal neovascularization and enhances physiological vascular regrowth in OIR as assessed by a new method of quantification. Invest. Ophthalmol. Vis. Sci. 47, 2125–2134 (2006).
Tzima, E. et al. Biologically active fragment of a human tRNA synthetase inhibits fluid shear stress–activated responses of endothelial cells. Proc. Natl. Acad. Sci. USA 100, 14903–14907 (2003).
Yao, P. et al. Coding region polyadenylation generates a truncated tRNA synthetase that counters translation repression. Cell 149, 88–100 (2012).
Han, J.M. et al. AIMP2/p38, the scaffold for the multi-tRNA synthetase complex, responds to genotoxic stresses via p53. Proc. Natl. Acad. Sci. USA 105, 11206–11211 (2008).
Choi, J.W. et al. AIMP2 promotes TNFα-dependent apoptosis via ubiquitin-mediated degradation of TRAF2. J. Cell Sci. 122, 2710–2715 (2009).
Choi, J.W. et al. Splicing variant of AIMP2 as an effective target against chemoresistant ovarian cancer. J. Mol. Cell Biol. 4, 164–173 (2012).
Choi, J.W. et al. Cancer-associated splicing variant of tumor suppressor AIMP2/p38: pathological implication in tumorigenesis. PLoS Genet. 7, e1001351 (2011).
Lee, J.W. et al. Editing-defective tRNA synthetase causes protein misfolding and neurodegeneration. Nature 443, 50–55 (2006).
Beebe, K., Ribas De Pouplana, L. & Schimmel, P. Elucidation of tRNA-dependent editing by a class II tRNA synthetase and significance for cell viability. EMBO J. 22, 668–675 (2003).
Antonellis, A. & Green, E.D. The role of aminoacyl-tRNA synthetases in genetic diseases. Annu. Rev. Genomics Hum. Genet. 9, 87–107 (2008).
Zhao, Z. et al. Alanyl-tRNA synthetase mutation in a family with dominant distal hereditary motor neuropathy. Neurology 78, 1644–1649 (2012).
Froelich, C.A. & First, E.A. Dominant intermediate Charcot-Marie-Tooth disorder is not due to a catalytic defect in tyrosyl-tRNA synthetase. Biochemistry 50, 7132–7145 (2011).
Xie, W., Nangle, L.A., Zhang, W., Schimmel, P. & Yang, X.L. Long-range structural effects of a Charcot-Marie-Tooth disease-causing mutation in human glycyl-tRNA synthetase. Proc. Natl. Acad. Sci. USA 104, 9976–9981 (2007).
Seburn, K.L., Nangle, L.A., Cox, G.A., Schimmel, P. & Burgess, R.W. An active dominant mutation of glycyl-tRNA synthetase causes neuropathy in a Charcot-Marie-Tooth 2D mouse model. Neuron 51, 715–726 (2006).
Stum, M. et al. An assessment of mechanisms underlying peripheral axonal degeneration caused by aminoacyl-tRNA synthetase mutations. Mol. Cell. Neurosci. 46, 432–443 (2011).
He, W. et al. Dispersed disease-causing neomorphic mutations on a single protein promote the same localized conformational opening. Proc. Natl. Acad. Sci. USA 108, 12307–12312 (2011).
van de Vijver, M.J. et al. A gene-expression signature as a predictor of survival in breast cancer. N. Engl. J. Med. 347, 1999–2009 (2002).
Paik, S. et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med. 351, 2817–2826 (2004).
Paley, E.L., Paley, D.E., Merkulova-Rainon, T. & Subbarayan, P.R. Hypoxia signature of splice forms of tryptophanyl-tRNA synthetase marks pancreatic cancer cells with distinct metastatic abilities. Pancreas 40, 1043–1056 (2011).
Ghanipour, A. et al. The prognostic significance of tryptophanyl-tRNA synthetase in colorectal cancer. Cancer Epidemiol. Biomarkers Prev. 18, 2949–2956 (2009).
Mun, J. et al. A proteomic approach based on multiple parallel separation for the unambiguous identification of an antibody cognate antigen. Electrophoresis 31, 3428–3436 (2010).
Kron, M.A., Petridis, M., Haertlein, M., Libranda-Ramirez, B. & Scaffidi, L.E. Do tissue levels of autoantigenic aminoacyl-tRNA synthetase predict clinical disease? Med. Hypotheses 65, 1124–1127 (2005).
Levine, S.M., Rosen, A. & Casciola-Rosen, L.A. Anti-aminoacyl tRNA synthetase immune responses: insights into the pathogenesis of the idiopathic inflammatory myopathies. Curr. Opin. Rheumatol 15, 708–713 (2003).
Park, S.G. et al. Autoantibodies against aminoacyl-tRNA synthetase: novel diagnostic marker for type 1 diabetes mellitus. Biomarkers 15, 358–366 (2010).
Mörbt, N. et al. Chlorinated benzenes cause concomitantly oxidative stress and induction of apoptotic markers in lung epithelial cells (A549) at nonacute toxic concentrations. J. Proteome Res. 10, 363–378 (2011).
Kim, S.S., Hur, S.Y., Kim, Y.R., Yoo, N.J. & Lee, S.H. Expression of AIMP1, 2 and 3, the scaffolds for the multi-tRNA synthetase complex, is downregulated in gastric and colorectal cancer. Tumori. 97, 380–385 (2011).
Yao, C. et al. P43/pro-EMAPII: a potential biomarker for discriminating traumatic versus ischemic brain injury. J. Neurotrauma 26, 1295–1305 (2009).
Park, M.C. et al. Secreted human glycyl-tRNA synthetase implicated in defense against ERK-activated tumorigenesis. Proc. Natl. Acad. Sci. USA 109, E640–E647 (2012).
Schwarz, R.E. et al. Antitumor effects of EMAP II against pancreatic cancer through inhibition of fibronectin-dependent proliferation. Cancer Biol. Ther. 9, 632–639 (2010).
Han, J.M., Park, S.G., Lee, Y. & Kim, S. Structural separation of different extracellular activities in aminoacyl-tRNA synthetase-interacting multi-functional protein, p43/AIMP1. Biochem. Biophys. Res. Commun. 342, 113–118 (2006).
Han, J.M. et al. Identification of gp96 as a novel target for treatment of autoimmune disease in mice. PLoS ONE 5, e9792 (2010).
Acknowledgements
This work was supported by US National Institutes of Health (NIH) grant GM 23562 and NCI grant CA92577 and by a fellowship from the US National Foundation for Cancer Research (P.S.), by NIH grant GM 100136, a Kimmel Scholar Award for Cancer Research and by funding from the state of Florida to Scripps Florida (M.G.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Guo, M., Schimmel, P. Essential nontranslational functions of tRNA synthetases. Nat Chem Biol 9, 145–153 (2013). https://doi.org/10.1038/nchembio.1158
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1158
This article is cited by
-
Impact of an oligosaccharide-based polymer on the metabolic profiles and microbial ecology of weanling pigs experimentally infected with a pathogenic E. coli
Journal of Animal Science and Biotechnology (2024)
-
Arginyl-tRNA synthetase in inflammation
Nature Cell Biology (2023)
-
A viral pan-end RNA element and host complex define a SARS-CoV-2 regulon
Nature Communications (2023)
-
Arg-tRNA synthetase links inflammatory metabolism to RNA splicing and nuclear trafficking via SRRM2
Nature Cell Biology (2023)
-
Tyrosyl-tRNA synthetase has a noncanonical function in actin bundling
Nature Communications (2023)