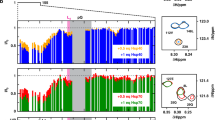
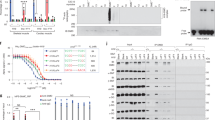
Abstract
We sought new strategies to reduce amounts of the polyglutamine androgen receptor (polyQ AR) and achieve benefits in models of spinobulbar muscular atrophy, a protein aggregation neurodegenerative disorder. Proteostasis of the polyQ AR is controlled by the heat shock protein 90 (Hsp90)- and Hsp70-based chaperone machinery, but mechanisms regulating the protein's turnover are incompletely understood. We demonstrate that overexpression of Hsp70 interacting protein (Hip), a co-chaperone that enhances binding of Hsp70 to its substrates, promotes client protein ubiquitination and polyQ AR clearance. Furthermore, we identify a small molecule that acts similarly to Hip by allosterically promoting Hsp70 binding to unfolded substrates. Like Hip, this synthetic co-chaperone enhances client protein ubiquitination and polyQ AR degradation. Both genetic and pharmacologic approaches targeting Hsp70 alleviate toxicity in a Drosophila model of spinobulbar muscular atrophy. These findings highlight the therapeutic potential of allosteric regulators of Hsp70 and provide new insights into the role of the chaperone machinery in protein quality control.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Zoghbi, H.Y. & Orr, H.T. Glutamine repeats and neurodegeneration. Annu. Rev. Neurosci. 23, 217–247 (2000).
Kennedy, W.R., Alter, M. & Sung, J.H. Progressive proximal spinal and bulbar muscular atrophy of late onset. A sex-linked recessive trait. Neurology 18, 671–680 (1968).
Sobue, G. et al. X-linked recessive bulbospinal neuronopathy. A clinicopathological study. Brain 112, 209–232 (1989).
Mhatre, A.N. et al. Reduced transcriptional regulatory competence of the androgen receptor in X-linked spinal and bulbar muscular atrophy. Nat. Genet. 5, 184–188 (1993).
Lieberman, A.P., Harmison, G., Strand, A.D., Olson, J.M. & Fischbeck, K.H. Altered transcriptional regulation in cells expressing the expanded polyglutamine androgen receptor. Hum. Mol. Genet. 11, 1967–1976 (2002).
Ranganathan, S. et al. Mitochondrial abnormalities in spinal and bulbar muscular atrophy. Hum. Mol. Genet. 18, 27–42 (2009).
McCampbell, A. et al. CREB-binding protein sequestration by expanded polyglutamine. Hum. Mol. Genet. 9, 2197–2202 (2000).
Yu, Z., Wang, A.M., Robins, D.M. & Lieberman, A.P. Altered RNA splicing contributes to skeletal muscle pathology in Kennedy disease knock-in mice. Dis. Model Mech. 2, 500–507 (2009).
Kemp, M.Q. et al. Impaired motoneuronal retrograde transport in two models of SBMA implicates two sites of androgen action. Hum. Mol. Genet. 20, 4475–4490 (2011).
Pratt, W.B. & Toft, D.O. Regulation of signaling protein function and trafficking by the hsp90/hsp70–based chaperone machinery. Exp. Biol. Med. (Maywood) 228, 111–133 (2003).
Pratt, W.B., Morishima, Y., Peng, H.M. & Osawa, Y. Proposal for a role of the Hsp90/Hsp70–based chaperone machinery in making triage decisions when proteins undergo oxidative and toxic damage. Exp. Biol. Med. (Maywood) 235, 278–289 (2010).
Pratt, W.B., Morishima, Y. & Osawa, Y. The Hsp90 chaperone machinery regulates signaling by modulating ligand binding clefts. J. Biol. Chem. 283, 22885–22889 (2008).
Thomas, M. et al. Pharmacologic and genetic inhibition of hsp90-dependent trafficking reduces aggregation and promotes degradation of the expanded glutamine androgen receptor without stress protein induction. Hum. Mol. Genet. 15, 1876–1883 (2006).
Tokui, K. et al. 17-DMAG ameliorates polyglutamine-mediated motor neuron degeneration through well-preserved proteasome function in a SBMA model mouse. Hum. Mol. Genet. 18, 898–910 (2009).
Waza, M. et al. 17-AAG, an Hsp90 inhibitor, ameliorates polyglutamine-mediated motor neuron degeneration. Nat. Med. 11, 1088–1095 (2005).
Kobayashi, Y. et al. Chaperones Hsp70 and Hsp40 suppress aggregate formation and apoptosis in cultured neuronal cells expressing truncated androgen receptor protein with expanded polyglutamine tract. J. Biol. Chem. 275, 8772–8778 (2000).
Bailey, C.K., Andriola, I.F., Kampinga, H.H. & Merry, D.E. Molecular chaperones enhance the degradation of expanded polyglutamine repeat androgen receptor in a cellular model of spinal and bulbar muscular atrophy. Hum. Mol. Genet. 11, 515–523 (2002).
Adachi, H. et al. Heat shock protein 70 chaperone overexpression ameliorates phenotypes of the spinal and bulbar muscular atrophy transgenic mouse model by reducing nuclear-localized mutant androgen receptor protein. J. Neurosci. 23, 2203–2211 (2003).
Adachi, H. et al. CHIP overexpression reduces mutant androgen receptor protein and ameliorates phenotypes of the spinal and bulbar muscular atrophy transgenic mouse model. J. Neurosci. 27, 5115–5126 (2007).
Howarth, J.L., Glover, C.P. & Uney, J.B. HSP70 interacting protein prevents the accumulation of inclusions in polyglutamine disease. J. Neurochem. 108, 945–951 (2009).
Höhfeld, J., Minami, Y. & Hartl, F.U. Hip, a novel cochaperone involved in the eukaryotic Hsc70/Hsp40 reaction cycle. Cell 83, 589–598 (1995).
Mayer, M.P., Brehmer, D., Gassler, C.S. & Bukau, B. Hsp70 chaperone machines. Adv. Protein Chem. 59, 1–44 (2001).
Buchberger, A. et al. Nucleotide-induced conformational changes in the ATPase and substrate binding domains of the DnaK chaperone provide evidence for interdomain communication. J. Biol. Chem. 270, 16903–16910 (1995).
Wang, A.M. et al. Inhibition of hsp70 by methylene blue affects signaling protein function and ubiquitination and modulates polyglutamine protein degradation. J. Biol. Chem. 285, 15714–15723 (2010).
Clapp, K.M. et al. C331A mutant of neuronal nitric-oxide synthase is labilized for Hsp70/CHIP (C terminus of HSC70-interacting protein)–dependent ubiquitination. J. Biol. Chem. 285, 33642–33651 (2010).
Peng, H.M. et al. Ubiquitylation of neuronal nitric-oxide synthase by CHIP, a chaperone-dependent E3 ligase. J. Biol. Chem. 279, 52970–52977 (2004).
Bender, A.T., Demady, D.R. & Osawa, Y. Ubiquitination of neuronal nitric-oxide synthase in vitro and in vivo. J. Biol. Chem. 275, 17407–17411 (2000).
Wadhwa, R. et al. Selective toxicity of MKT-077 to cancer cells is mediated by its binding to the hsp70 family protein mot-2 and reactivation of p53 function. Cancer Res. 60, 6818–6821 (2000).
Tikoo, A. et al. Treatment of ras-induced cancers by the F-actin–bundling drug MKT-077. Cancer J. 6, 162–168 (2000).
Rousaki, A. et al. Allosteric drugs: the interaction of antitumor compound MKT-077 with human Hsp70 chaperones. J. Mol. Biol. 411, 614–632 (2011).
Morishima, Y. et al. Heme-dependent activation of neuronal nitric oxide synthase by cytosol is due to an Hsp70-dependent, thioredoxin-mediated thiol-disulfide interchange in the heme/substrate binding cleft. Biochemistry 50, 7146–7156 (2011).
Takeyama, K. et al. Androgen-dependent neurodegeneration by polyglutamine-expanded human androgen receptor in Drosophila. Neuron 35, 855–864 (2002).
Burlison, J.A., Neckers, L., Smith, A.B., Maxwell, A. & Blagg, B.S. Novobiocin: redesigning a DNA gyrase inhibitor for selective inhibition of hsp90. J. Am. Chem. Soc. 128, 15529–15536 (2006).
Evans, C.G., Chang, L. & Gestwicki, J.E. Heat shock protein 70 (hsp70) as an emerging drug target. J. Med. Chem. 53, 4585–4602 (2010).
Patury, S., Miyata, Y. & Gestwicki, J.E. Pharmacological targeting of the Hsp70 chaperone. Curr. Top. Med. Chem. 9, 1337–1351 (2009).
Roodveldt, C. et al. Chaperone proteostasis in Parkinson′s disease: stabilization of the Hsp70/α-synuclein complex by Hip. EMBO J. 28, 3758–3770 (2009).
Shorter, J. The mammalian disaggregase machinery: Hsp110 synergizes with Hsp70 and Hsp40 to catalyze protein disaggregation and reactivation in a cell-free system. PLoS ONE 6, e26319 (2011).
Peng, H.-M., Morishima, Y., Pratt, W.B. & Osawa, Y. Modulation of the heme/substrate-binding cleft of neuronal nitric-oxide synthase regulates binding of Hsp90 and Hsp70 and nNOS ubiquitination. J. Biol. Chem. 287, 1556–1565 (2012).
Morishima, Y. et al. CHIP deletion reveals functional redundancy of E3 ligases in promoting degradation of both signaling proteins and expanded glutamine proteins. Hum. Mol. Genet. 17, 3942–3952 (2008).
Baldo, B. et al. A screen for enhancers of clearance identifies huntingtin as an heat shock protein 90 (Hsp90) client protein. J. Biol. Chem. 287, 1406–1414 (2012).
Petrucelli, L. et al. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum. Mol. Genet. 13, 703–714 (2004).
Dou, F. et al. Chaperones increase association of tau protein with microtubules. Proc. Natl. Acad. Sci. USA 100, 721–726 (2003).
Walcott, J.L. & Merry, D.E. Ligand promotes intranuclear inclusions in a novel cell model of spinal and bulbar muscular atrophy. J. Biol. Chem. 277, 50855–50859 (2002).
Chang, L. et al. Chemical screens against a reconstituted multiprotein complex: myricetin blocks DnaJ regulation of DnaK through an allosteric mechanism. Chem. Biol. 18, 210–221 (2011).
Chang, L., Thompson, A.D., Ung, P., Carlson, H.A. & Gestwicki, J.E. Mutagenesis reveals the complex relationships between ATPase rate and the chaperone activities of Escherichia coli heat shock protein 70 (Hsp70/DnaK). J. Biol. Chem. 285, 21282–21291 (2010).
Kanelakis, K.C. et al. hsp70 interacting protein Hip does not affect glucocorticoid receptor folding by the hsp90-based chaperone machinery except to oppose the effect of BAG-1. Biochemistry 39, 14314–14321 (2000).
Miyata, Y. et al. High-throughput screen for Escherichia coli heat shock protein 70 (Hsp70/DnaK): ATPase assay in low volume by exploiting energy transfer. J. Biomol. Screen. 15, 1211–1219 (2010).
Budnik, V. et al. Regulation of synapse structure and function by the Drosophila tumor suppressor gene dlg. Neuron 17, 627–640 (1996).
Freeman, M. Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell 87, 651–660 (1996).
Mahr, A. & Aberle, H. The expression pattern of the Drosophila vesicular glutamate transporter: a marker protein for motoneurons and glutamatergic centers in the brain. Gene Expr. Patterns 6, 299–309 (2006).
Kawakami, M. et al. Structure-activity of novel rhodacyanine dyes as antitumor agents. J. Med. Chem. 41, 130–142 (1998).
Acknowledgements
We thank S. Reddy, J. Diep and T. Washington for technical assistance and K. Takeyama (University of Tokyo) and the Bloomington Stock Center for fly strains. This work was initiated under a grant from the McKnight Foundation (to A.P.L.) and was supported by the US National Institutes of Health (NS055746, NS055746-04S1 to A.P.L.; NS059690 to J.E.G.; GM077430 to Y.O.; NS069844 to C.A.C.; NS32214 to D.E.M.; NS076189 to J.P.C.), the Muscular Dystrophy Association (MDA238924 to A.P.L.) and the US National Science Foundation (IOS-0842701 to C.A.C.).
Author information
Authors and Affiliations
Contributions
A.M.W., S.K., Y. Miyata, H.-M.P., J.P.C., W.B.P., Y.O., C.A.C., J.E.G. and A.P.L. designed the experiments. A.M.W., S.K., Y. Miyata, H.-M.P., J.P.C., T.K., X.L. and Y. Morishima performed the experiments. A.M.W., S.K., Y. Miyata, H.-M.P., J.P.C., T.K., W.B.P., Y.O., C.A.C., J.E.G. and A.P.L. interpreted the data. Y.O., J.E.G. and D.E.M. contributed reagents or analytical tools. A.M.W., W.B.P., J.E.G. and A.P.L. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results (PDF 9172 kb)
Rights and permissions
About this article
Cite this article
Wang, A., Miyata, Y., Klinedinst, S. et al. Activation of Hsp70 reduces neurotoxicity by promoting polyglutamine protein degradation. Nat Chem Biol 9, 112–118 (2013). https://doi.org/10.1038/nchembio.1140
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1140
This article is cited by
-
HSP70-binding motifs function as protein quality control degrons
Cellular and Molecular Life Sciences (2023)
-
Expanding the role of proteasome homeostasis in Parkinson’s disease: beyond protein breakdown
Cell Death & Disease (2021)
-
Targeting Hsp70 facilitated protein quality control for treatment of polyglutamine diseases
Cellular and Molecular Life Sciences (2020)
-
MEF2 impairment underlies skeletal muscle atrophy in polyglutamine disease
Acta Neuropathologica (2020)
-
Side chain to main chain hydrogen bonds stabilize a polyglutamine helix in a transcription factor
Nature Communications (2019)