Abstract
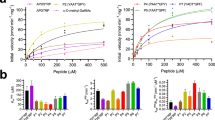
Visualization of the reaction coordinate undertaken by glycosyltransferases has remained elusive but is critical for understanding this important class of enzyme. Using substrates and substrate mimics, we describe structural snapshots of all species along the kinetic pathway for human O-linked β-N-acetylglucosamine transferase (O-GlcNAc transferase), an intracellular enzyme that catalyzes installation of a dynamic post-translational modification. The structures reveal key features of the mechanism and show that substrate participation is important during catalysis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Lairson, L.L., Henrissat, B., Davies, G.J. & Withers, S.G. Annu. Rev. Biochem. 77, 521–555 (2008).
Torres, C.R. & Hart, G.W. J. Biol. Chem. 259, 3308–3317 (1984).
Kreppel, L.K., Blomberg, M.A. & Hart, G.W. J. Biol. Chem. 272, 9308–9315 (1997).
Sinclair, D.A. et al. Proc. Natl. Acad. Sci. USA 106, 13427–13432 (2009).
Love, D.C. et al. Proc. Natl. Acad. Sci. USA 107, 7413–7418 (2010).
Kazemi, Z., Chang, H., Haserodt, S., McKen, C. & Zachara, N.E. J. Biol. Chem. 285, 39096–39107 (2010).
Dentin, R., Hedrick, S., Xie, J., Yates, J. III & Montminy, M. Science 319, 1402–1405 (2008).
Lazarus, M.B., Nam, Y., Jiang, J., Sliz, P. & Walker, S. Nature 469, 564–567 (2011).
Gloster, T.M. et al. Nat. Chem. Biol. 7, 174–181 (2011).
Iyer, S.P. & Hart, G.W. J. Biol. Chem. 278, 24608–24616 (2003).
Gross, B.J., Kraybill, B.C. & Walker, S. J. Am. Chem. Soc. 127, 14588–14589 (2005).
Lee, S.S. et al. Nat. Chem. Biol. 7, 631–638 (2011).
Burkart, M.D. et al. Bioorg. Med. Chem. 8, 1937–1946 (2000).
Vocadlo, D.J. & Davies, G.J. Curr. Opin. Chem. Biol. 12, 539–555 (2008).
Schramm, V.L. & Shi, W. Curr. Opin. Struct. Biol. 11, 657–665 (2001).
Vocadlo, D.J., Davies, G.J., Laine, R. & Withers, S.G. Nature 412, 835–838 (2001).
Davies, G.J., Planas, A. & Rovira, C. Acc. Chem. Res. 45, 308–316 (2012).
Martinez-Fleites, C. et al. Nat. Struct. Mol. Biol. 15, 764–765 (2008).
Dorfmueller, H.C. et al. Amino Acids 40, 781–792 (2011).
Errey, J.C. et al. Angew. Chem. Int. Edn Engl. 49, 1234–1237 (2010).
Reinert, D.J., Jank, T., Aktories, K. & Schulz, G.E. J. Mol. Biol. 351, 973–981 (2005).
Shen, D.L., Gloster, T.M., Yuzwa, S.A. & Vocadlo, D.J. J. Biol. Chem. 287, 15395–15408 (2012).
Ramakrishnan, B., Ramasamy, V. & Qasba, P.K. J. Mol. Biol. 357, 1619–1633 (2006).
Persson, K. et al. Nat. Struct. Biol. 8, 166–175 (2001).
Ni, L. et al. Biochemistry 46, 6288–6298 (2007).
Acknowledgements
This work was supported by the US National Institutes of Health (NIH) grant R01GM094263 to S.W. and the Natural Sciences and Engineering Research Council of Canada and Simon Fraser University. T.M.G. is a Sir Henry Wellcome postdoctoral fellow and a Michael Smith for Health Research (MSFHR) trainee award holder. D.J.V. is an E.W.R. Steacie Memorial Fellow and holds a Canada Research Chair in Chemical Glycobiology. We thank L. Deng for assistance with the chemical synthesis. We thank L. Cai (University of South Carolina, Salkehatchie) for the kind gift of UDP-2-ketoGlc.
Author information
Authors and Affiliations
Contributions
M.B.L. carried out all structural experiments. J.J. helped with X-ray data collection. J.J. and M.B.L. performed kinetics assays. W.F.Z. and T.M.G. performed thiosugar kinetics assays and peptide assays. G.E.W. performed the synthesis of peptide and glycopeptides substrates. M.B.L., J.J., T.M.G., D.J.V. and S.W. designed experiments, analyzed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Results (PDF 3577 kb)
Rights and permissions
About this article
Cite this article
Lazarus, M., Jiang, J., Gloster, T. et al. Structural snapshots of the reaction coordinate for O-GlcNAc transferase. Nat Chem Biol 8, 966–968 (2012). https://doi.org/10.1038/nchembio.1109
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1109
This article is cited by
-
Optimizing the binding of OGT and a peptidic substrate towards pseudo-substrate inhibitors via molecular dynamic simulations
Systems Microbiology and Biomanufacturing (2024)
-
Molecular basis for bacterial N-glycosylation by a soluble HMW1C-like N-glycosyltransferase
Nature Communications (2023)
-
Glycosyltransferases as targets for therapeutic intervention in cancer and inflammation: molecular modeling insights
Chemical Papers (2022)
-
Identification of UAP1L1 as a critical factor for protein O-GlcNAcylation and cell proliferation in human hepatoma cells
Oncogene (2019)
-
Emerging structural insights into glycosyltransferase-mediated synthesis of glycans
Nature Chemical Biology (2019)