Abstract
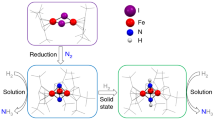
Nitric oxide, NO, the diatomic hybrid of dinitrogen and dioxygen, has extensive biochemical, industrial and atmospheric chemistry. The unpaired electron on NO makes it highly reactive and its facile oxidation and reduction to make (NO)1+ and (NO)1−, respectively, have been heavily studied. Now the (NO)2− dianion has been isolated for the first time from the two-electron reduction of NO by the recently discovered (N2)3− yttrium complex {[(Me3Si)2N]2(THF)Y}2(µ3-η2:η2:η2-N2)K. NO reacts with this complex to form {[(Me3Si)2N]2(THF)Y}2(µ-η2:η2-NO), a paramagnetic complex that has an electron paramagnetic resonance spectrum definitive for the (NO)2− radical. Density functional theory reveals that a metal dπ to ligand π* interaction is crucial for the stability of this complex, which reacts with additional NO to generate the diamagnetic (ON=NO)2− product, {[(Me3Si)2N]2Y}4(µ3-ON=NO)2(THF)2.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Koshland, Jr., D. E. The molecule of the year. Science 258, 1861 (1992).
Gray, H. B., Simon, J. D. & Trogler, W. C. Braving the Elements (University Science Books, 1995).
Schwartz, S. E. & White, W. H. Trace Atmospheric Constituents: Properties, Transformations, and Fates (John Wiley & Sons, 1983).
Ignarro, L. J. Nitric Oxide: Biology and Pathobiology (Academic Press, 2000).
Feelisch, M. & Stamler, J. S. Methods in Nitric Oxide Research (John Wiley and Sons, 1996).
Butler, A. R. & Williams, D. L. H. The physiological role of nitric oxide. Chem. Soc. Rev. 22, 233–241 (1993).
Moncada, S., Palmer, R. M. J. & Higgs, E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 43, 109–142 (1991).
Palmer, R. M. J., Ferrige, A. G. & Moncada, S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327, 524–526 (1987).
Gusarov, I., Shatalin, K., Starodubtseva, M. & Nudler, E. Endogenous nitric oxide protects bacteria against a wide spectrum of antibiotics. Science 325, 1380–1384 (2009).
McCleverty, J. A. Chemistry of nitric oxide relevant to biology. Chem. Rev. 104, 403–418 (2004).
Ford, P. C. & Lorkovic, I. M. Mechanistic aspects of the reactions of nitric oxide with transition-metal complexes. Chem. Rev. 102, 993–1018 (2002).
Farmer, P. J. & Sulc, F. Coordination chemistry of the HNO ligand with hemes and synthetic coordination complexes. J. Inorg. Biochem. 99, 166–184 (2005).
Montenegro, A. C. et al. Three redox states of nitrosyl: NO+, NO., and NO−/HNO interconvert reversibly on the same pentacyanoferrate(II) platform. Angew. Chem. Int. Ed. 48, 4213–4216 (2009).
Stamler, J. S., Singel, D. J. & Loscalzo, J. Biochemistry of nitric oxide and its redox-activated forms. Science 258, 1898–1902 (1992).
Evans, W. J. et al. Isolation of dysprosium and yttrium complexes of a three-electron reduction product in the activation of dinitrogen, the (N2)3− radical. J. Am. Chem. Soc. 131, 11195–11202 (2009).
Kaim, W. & Sarkar, B. N2.3-: filling a gap in the N2n- series. Angew. Chem. Int. Ed. 48, 9409–9411 (2009).
Evans, W. J. & Lee, D. S. Early developments in lanthanide-based dinitrogen reduction chemistry. Can. J. Chem. 83, 375–384 (2005).
Evans, W. J., Lee, D. S., Lie, C. & Ziller, J. W. Expanding the LnZ3/alkali-metal reduction system to organometallic and heteroleptic precursors: formation of dinitrogen derivatives of lanthanum. Angew. Chem. Int. Ed. 43, 5517–5519 (2004).
Evans, W. J. et al. Expanding dinitrogen reduction chemistry to trivalent lanthanides via the LnZ3/alkali metal reduction system: evaluation of the generality of forming Ln2(μ-η2:η2-N2) complexes via LnZ3/K. J. Am. Chem. Soc. 126, 14574–14582 (2004).
Evans, W. J., Lee, D. S., Ziller, J. W. & Kaltsoyannis, N. Trivalent [(C5Me5)2(THF)Ln]2(μ-η2:η2-N2) complexes as reducing agents including the reductive homologation of CO to a ketene carboxylate, (μ-η4-O2C-C=C=O)2-. J. Am. Chem. Soc. 128, 14176–14184 (2006).
Evans, W. J., Lorenz, S. E. & Ziller, J. W. Investigating metal size effects in the Ln2(μ-η2:η2-N2) reduction system: reductive reactivity with complexes of the largest and smallest trivalent lanthanide ions, La3+ and Lu3+. Inorg. Chem. 48, 2001–2009 (2009).
Krynitz, U., Gerson, F., Wiberg, N. & Veith, M. ESR spectra of radical anions of aliphatic azo compounds: tert-butyl and trimethylsilyl derivatives of diimine. Angew. Chem. Int. Ed. 8, 755–756 (1969).
Bau, R., Sabherwal, I. H. & Burg, A. B. Preparation and structure of Co4(NO)8(NO2)2(N2O2). Novel complex containing a quadridentate hyponitrite group. J. Am. Chem. Soc. 93, 4926–4928 (1971).
Arulsamy, N., Bohle, D. S., Imonigie, J. A. & Levine, S. An umpolung approach to cis-hyponitrite complexes. Angew. Chem. Int. Ed. 41, 2371–2373 (2002).
Bottcher, H.-C., Graf, M., Mereiter, K. & Kirchner, K. Reductive dimerization of nitric oxide to trans-hyponitrite in the coordination sphere of a dinuclear ruthenium complex. Organometallics 23, 1269–1273 (2004).
Villalba, M. E. C., Navaza, A., Güida, J. A., Varetti, E. L. & Aymonino, P. J. New structural study and reinterpretation of the vibrational spectra of the µ-N, O-hyponitrite bis[pentaamminecobalt(III)]4+ cation. Inorg. Chim. Acta 359, 707–712 (2006).
Arulsamy, N., Bohle, D. S., Imonigie, J. A. & Moore, R. C. Group 8 and 10 hyponitrite and dinitrosyl complexes. Polyhedron 26, 4737–4745 (2007).
Xu, N., Campbell, A. L. O., Powell, D. R., Khandogin, J. & Richter-Addo, G. B. A stable hyponitrite-bridged iron porphyrin complex. J. Am. Chem. Soc. 131, 2460–2461 (2009).
Černák, J. et al. Cyanocomplexes with one-dimensional structures: preparations, crystal structures and magnetic properties. Coord. Chem. Rev. 224, 51–66 (2002).
Daff, P. J., Legzdins, P. & Rettig, S. J. The first nitrosyl complexes of niobium and tantalum. J. Am. Chem. Soc. 120, 2688–2689 (1998).
Richter-Addo, G. B. & Legzdins, P. Metal Nitrosyls (Oxford University Press, 1992).
Huber, K. P. & Herzberg, G. Constants of diatomic molecules (data prepared by Gallagher, J. W. & Johnson, R. D. III) in NIST Chemistry WebBook, NIST Standard Reference Database Number 69 (eds Linstrom, P. J. & Mallard, W. G.) (National Institute of Standards and Technology, 2009).
Zuo, T. et al. M2@C79N (M=Y, Tb): isolation and characterization of stable endohedral metallofullerenes exhibiting M-M bonding interactions inside aza[80]fullerene cages. J. Am. Chem. Soc. 130, 12992–12997 (2008).
Acknowledgements
We thank the US National Science Foundation for support of this research (CHE 0703372, 0723168, and 0809384), D. C. Lacy, E. Fadeev, M. J. Nilges and A. S. Borovik for help with EPR spectral studies, M. K. Takase for help with X-ray crystallography studies, and T. J. Mueller for assistance with the elemental analysis.
Author information
Authors and Affiliations
Contributions
W.J.E. and M.F. designed the study. Crystallography was done by J.W.Z. DFT calculations were done by J.E.B. and F.F. Raman spectra were obtained by M.D.K. and J.I.Z. All other experimental research was done by M.F. W.J.E. wrote the manuscript, which was reviewed and edited by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 561 kb)
Rights and permissions
About this article
Cite this article
Evans, W., Fang, M., Bates, J. et al. Isolation of a radical dianion of nitrogen oxide (NO)2−. Nature Chem 2, 644–647 (2010). https://doi.org/10.1038/nchem.701
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.701