Abstract
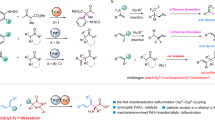
Stereoselective functionalizations of organic molecules are of great importance to modern synthesis. A stereoselective preparation of pharmaceutically active molecules is often required to ensure the appropriate biological activity. Thereby, diastereoselective methods represent valuable tools for an efficient set-up of multiple stereocentres. In this article, highly diastereoselective Csp3 Negishi cross-couplings of various cycloalkylzinc reagents with aryl halides are reported. In all cases, the thermodynamically most-stable stereoisomer was obtained. Remarkably, this diastereoselective coupling was successful not only for 1,2-substituted cyclic systems, but also for 1,3- and 1,4-substituted cyclohexylzinc reagents. The origin of this remote stereocontrol was investigated by NMR experiments and density functional theory calculations. A detailed mechanism based on these experimental and theoretical data is proposed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Rudolph, A. & Lautens, M. Secondary alkyl halides in transition-metal-catalyzed cross-coupling reactions. Angew. Chem. Int. Ed. 48, 2656–2670 (2009).
Terao, J. & Kambe, N. Cross-coupling reaction of alkyl halides with Grignard reagents catalyzed by Ni, Pd, or Cu complexes with π-carbon ligand(s). Acc. Chem. Res. 41, 1545–1554 (2008).
Chemler, S. R., Trauner, D. & Danishefsky, S. J. The β-alkyl Suzuki–Miyaura cross-coupling reaction: development, mechanistic study, and applications in natural product synthesis. Angew. Chem. Int. Ed. 40, 4544–4568 (2001).
Corbet, J.-P. & Mignani, G. Selected patented cross-coupling reaction technologies. Chem. Rev. 106, 2651–2710 (2006).
Valente, C., Baglione, S., Candito, D., O'Brien, C. J. & Organ, M. G. High yielding alkylations of unactivated sp3 and sp2 centers with alkyl-9-BBN reagents using an NHC-based catalyst: Pd-PEPPSI-IPr. Chem. Commun. 735–737 (2008).
Jin, L., Zhao, Y., Wang, H. & Lei, A. Palladium-catalyzed R(sp3)–Zn/R(sp)–SnBu3 oxidative cross-coupling. Synthesis 649–654 (2008).
Zhao, Y. et al. Oxidative cross-coupling through double transmetallation: surprisingly high selectivity for palladium-catalyzed cross-coupling of alkylzinc and alkynylstannanes. J. Am. Chem. Soc. 128, 15048–15049 (2006).
Hadei, N., Kantchev, E. A. B., O'Brien, C. J. & Organ, M. G. The first Negishi cross-coupling reaction of two alkyl centers utilizing a Pd–N–heterocyclic carbene (NHC) catalyst. Org. Lett. 7, 3805–3807 (2005).
Powell, D. A., Maki, T. & Fu, G. C. Stille cross-couplings of unactivated secondary alkyl halides using monoorganotin reagents. J. Am Chem. Soc. 127, 510–511 (2005).
Negishi, E., Valente, L. F. & Kobayashi, M. Palladium-catalyzed cross-coupling reaction of homoallylic or homopropargylic organozincs with alkenyl halides as a new selective route to 1,5-dienes and 1,5-enynes. J. Am Chem. Soc. 102, 3298–3299 (1980).
Hayashi, T., Konishi, M. & Kumada, M. Dichloro[1,1′-bis(diphenylphosphino)ferrocene]-palladium(ii): an effective catalyst for cross-coupling reaction of a secondary alkyl Grignard reagent with organic halides. Tetrahedron Lett. 1871–1874 (1979).
Studte, C. & Breit B. Zinc-catalyzed enantiospecific sp3–sp3 cross-coupling of alpha-hydroxy ester triflates with Grignard reagents. Angew. Chem. Int. Ed. 47, 5451–5455 (2008).
Rodriguez, N. et al. Palladium-catalyzed reaction of boronic acids with chiral and racemic α-bromo sulfoxides. J. Org. Chem. 69, 8070–8076 (2004).
Hoelzer, B. & Hoffmann, R. W. Kumada–Corriu coupling of Grignard reagents, probed with a chiral Grignard reagent. Chem. Commun. 732–733 (2003).
Glorius, F. Asymmetric cross-coupling of non-activated secondary alkyl halides. Angew. Chem. Int. Ed. 47, 8347–8349 (2008).
Hayashi, T. Catalytic asymmetric cross-coupling. J. Organomet. Chem. 653, 41–45 (2002).
Cross, G., Vriesema, B. K., Boven, G., Kellogg, R. M. & Van Bolhuis, F. Transition-metal-catalyzed asymmetric cross coupling reactions. New ligands and the effects of added salts. Crystal structures of [Ph2PCH2CH{(CH2)3SMe}NMe2]PdCl2 and [Ph2PCH2CH{(CH2)2SMe}NMe2]PdCl2 . J. Organomet. Chem. 370, 357–381 (1989).
Hayashi, T., Hagihara, T., Katsuro, Y. & Kumada, M. Asymmetric cross-coupling of organozinc reagents with alkenyl bromides catalyzed by a chiral ferrocenylphosphine–palladium complex. Bull. Chem. Soc. Jpn 56, 363–364 (1983).
Lundin, P. M., Esquivias, J. & Fu, G. C. Catalytic asymmetric cross-couplings of racemic α-bromoketones with arylzinc reagents. Angew. Chem. Int. Ed. 48, 154–156 (2009).
Caeiro, J., Perez Sestelo, J. & Sarandeses, L. A. Enantioselective nickel-catalyzed cross-coupling reactions of trialkynylindium reagents with racemic secondary benzyl bromides. Chem. Eur. J. 14, 741–746 (2008).
Fischer, C. & Fu, G. C. Asymmetric nickel-catalyzed Negishi cross-couplings of secondary α-bromo amides with organozinc reagents. J. Am. Chem. Soc. 127, 4594–4595 (2005).
Hayashi, T. et al. Preparation of (R)-N,N-dimethyl-1-[2-(diphenylphosphino)ferrocenyl]-2-propanamines and asymmetric Grignard cross-coupling catalyzed by nickel complexes with the phosphine ligands. Bull. Chem. Soc. Jpn 54, 3615–3616 (1981).
Hayashi, T., Tajika, M., Tamao, K. & Kumada, M. High stereoselectivity in asymmetric Grignard cross-coupling catalyzed by nickel complexes of chiral (aminoalkylferrocenyl)phosphines. J. Am. Chem. Soc. 98, 3718–3719 (1976).
Negishi, E., King, A. O. & Okukado, N. Selective carbon–carbon bond formation via transition metal catalysis. 3. A highly selective synthesis of unsymmetrical biaryls and diarylmethanes by the nickel- or palladium-catalyzed reaction of aryl- and benzylzinc derivatives with aryl halides. J. Org. Chem. 42, 1821–1823 (1977).
Beckmann, J., Dakternieks, D., Draeger, M. & Duthie, A. New insights into the classic chiral Grignard reagent (1R,2S,5R)-menthylmagnesium chloride. Angew. Chem. Int. Ed. 45, 6509–6512 (2006).
Lange, G. L. & Gottardo, C. Facile conversion of primary and secondary alcohols to alkyl iodides. Synth. Commun. 20, 1473–1479 (1990).
Krasovskiy, A., Malakhov, V., Gavryushin, A. & Knochel, P. Efficient synthesis of functionalized organozinc compounds by the direct insertion of zinc into organic iodides and bromides. Angew. Chem. Int. Ed. 45, 6040–6044 (2006).
Walker, S. D., Barder, T. E., Martinelli, J. R. & Buchwald, S. L. A rationally designed universal catalyst for Suzuki–Miyaura coupling processes. Angew. Chem. Int. Ed. 43, 1871–1876 (2004).
Barnier, J. P. & Blanco, L. Chemo-enzymatic preparations of (R)- and (S)-3-iodocyclohex-2-en-1-yl acetate. Synth. Commun. 33, 2487–2496 (2003).
Dreher, S. D., Dormer, P. G., Sandrock, D. L. & Molander, G. A. Efficient cross-coupling of secondary alkyltrifluoroborates with aryl chlorides – reaction discovery using parallel microscale experimentation. J. Am. Chem. Soc. 130, 9257–9259 (2008).
Dunbar, K. R. & Sun, J.-S. Synthesis and structure of the distorted octahedral palladium(ii) complex [Pd(tmpp)2][BF4]2 [tmpp = tris(2,4,6-trimethoxyphenyl)phosphine]. J. Chem. Soc. Chem. Commun. 2387–2388 (1994).
Scianowski, J., Rafinski, Z. & Wojtczak, A. Syntheses and reactions of new optically active terpene dialkyl diselenides. Eur. J. Org. Chem. 3216–3225 (2006).
Kosower, E. M. & Winstein, S. Neighboring carbon and hydrogen. XXIII. Homoallylic systems. 3,5-Cyclocholestan-6β-yl chloride. J. Am. Chem. Soc. 78, 4354–4358 (1956).
Hirsch, R. & Hoffmann, R. W. Chiral organometallic reagents. V. A test on the configurational stability of chiral organolithium compounds based on kinetic resolution; scope and limitations. Chem. Ber. 125, 975–982 (1992).
Guijarro, A. & Rieke, R. D. Study of the configuration stability of the carbon–zinc bond, direct measurement of enantiomeric ratios, and tentative assignment of the absolute configuration in secondary organozinc halides. Angew. Chem. Int. Ed. 39, 1475–1479 (2000).
Micouin, L., Oestreich, M. & Knochel, P. Stereoselective preparation and reactions of cycloalkylzinc compounds. Angew. Chem. Int. Ed. 36, 245–246 (1997).
Boudier, A. et al. Stereoselective preparation and reactions of configurationally defined dialkylzinc compounds. Chem. Eur. J. 6, 2748–2761 (2000).
Campos, K. R., Klapars, A., Waldman, J. H., Dormer, P. G. & Chen, C.-y. Enantioselective, palladium-catalyzed α-arylation of N-Boc-pyrrolidine. J. Am. Chem. Soc. 128, 3538–3539 (2006).
Frisch, M. J. et al. Gaussian 03 (Gaussian Inc., Wallingford, Connecticut, 2004).
Parr, R. G. & Yang, W. Density Functional Theory of Atoms and Molecules (Oxford Univ. Press, 1989).
Ziegler, T. Approximate density functional theory as a practical tool in molecular energetics and dynamics. Chem. Rev. 91, 651–667 (1991).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Becke, A. D. Density functional exchange-energy approximation with correct asymptotic behaviour. Phys. Rev. A 38, 3098–3100 (1988).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Hariharan, P. C. & Pople, J. A. The influence of polarization functions on molecular orbital hydrogenation energies. Theoret. Chim. Acta 28, 213–222 (1973).
Francl, M. M. et al. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 77, 3654–3665 (1982).
Rassolov, V. A., Pople, J. A., Ratner, M. A. & Windus, T. L. 6-31G* basis set for atoms K through Zn. J. Chem. Phys. 109, 1223–1229 (1998).
Acknowledgements
We thank the European Research Council, the Fonds der Chemischen Industrie, the Sonderforschungsbereich 749 and the Deutsche Forschungsgemeinschaft for financial support. We also thank Evonik Degussa GmbH, BASF AG, W. C. Heraeus GmbH, Chemetall GmbH and Solvias AG for generous gifts of chemicals.
Author information
Authors and Affiliations
Contributions
T.T. and A.G. planned, conducted and analysed the experiments. B.H. and H.Z. planned and analysed the DFT calculations. B.H. conducted the DFT calculations. T.T., K.S., E.H. and R.M.G. planned and conducted the NMR experiments. K.S., E.H. and R.M.G. analysed the NMR experiments. P.M. performed the X-ray analysis. P.K. designed and directed the project and wrote the manuscript, with contributions from T.T., B.H. and R.M.G. All authors except P.M. contributed to discussions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 4552 kb)
Supplementary information
Crystallographic data for compound 5a (CIF 16 kb)
Supplementary information
Crystallographic data for compound 18a (CIF 17 kb)
Supplementary information
Crystallographic data for compound 31 (CIF 42 kb)
Rights and permissions
About this article
Cite this article
Thaler, T., Haag, B., Gavryushin, A. et al. Highly diastereoselective Csp3–Csp2 Negishi cross-coupling with 1,2-, 1,3- and 1,4-substituted cycloalkylzinc compounds. Nature Chem 2, 125–130 (2010). https://doi.org/10.1038/nchem.505
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.505
This article is cited by
-
A modular synthesis of tetracyclic meroterpenoid antibiotics
Nature Communications (2017)
-
Nickel-Catalyzed Reductive Couplings
Topics in Current Chemistry (2016)
-
Stereoretentive Pd-catalysed Stille cross-coupling reactions of secondary alkyl azastannatranes and aryl halides
Nature Chemistry (2013)
-
Stereoselective C(sp3)-C(sp2) Negishi coupling of (2-amido-1-phenylpropyl)zinc compounds through the steric control of β-amido group
Science China Chemistry (2013)
-
Stereoselective palladium-catalyzed cross-coupling of (2-amido-1-phenylpropyl)zinc compounds with aryl bromides
Chemical Research in Chinese Universities (2013)