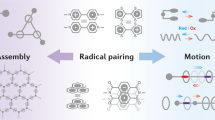
Abstract
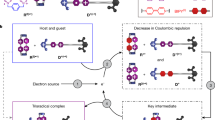
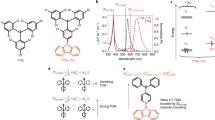
The tendency for viologen radical cations to dimerize has been harnessed to establish a recognition motif based on their ability to form extremely strong inclusion complexes with cyclobis(paraquat-p-phenylene) in its diradical dicationic redox state. This previously unreported complex involving three bipyridinium cation radicals increases the versatility of host–guest chemistry, extending its practice beyond the traditional reliance on neutral and charged guests and hosts. In particular, transporting the concept of radical dimerization into the field of mechanically interlocked molecules introduces a higher level of control within molecular switches and machines. Herein, we report that bistable and tristable [2]rotaxanes can be switched by altering electrochemical potentials. In a tristable [2]rotaxane composed of a cyclobis(paraquat-p-phenylene) ring and a dumbbell with tetrathiafulvalene, dioxynaphthalene and bipyridinium recognition sites, the position of the ring can be switched. On oxidation, it moves from the tetrathiafulvalene to the dioxynaphthalene, and on reduction, to the bipyridinium radical cation, provided the ring is also reduced simultaneously to the diradical dication.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Jeon, W. S., Kim, H.-J., Lee, C. & Kim, K. Control of the stoichiometry in host-guest complexation by redox chemistry of guests: Inclusion of methylviologen in cucurbit[8]uril. Chem. Commun. 1828–1829 (2002).
Hwang, I., Ziganshina, A. Y., Ko, Y. H., Yun, G. & Kim, K. A. new three-way supramolecular switch based on redox-controlled interconversion of heter- and homo-guest-pair inclusion inside a host molecule. Chem. Commun. 416–418 (2008).
Kim, K. et al. Functionalized cucurbiturils and their applications. Chem. Soc. Rev. 36, 267–279 (2007).
Ko, Y. H., Kim, E., Hwang, I. & Kim, K. Supramolecular assemblies built with host-stabilized charge-transfer interactions. Chem. Commun. 1305–1315 (2007).
Goren, Z. & Willner, I. Photochemical and chemical reduction of vicinal dibromides via phase transfer of 4,4'-bipyridinium radical: the role of radical disproportionation. J. Am. Chem. Soc. 105, 7764–7765 (1983).
Endo, T., Saotome, Y. & Okawara, M. Viologens used ‘electron phase transfer’. Catalytic debromination of vic-dibromides under heterophase conditions using viologens J. Am. Chem. Soc. 106, 1124–1125 (1984).
Park, K. K., Oh, C. H. & Joung, W. K. Sodium dithionite reduction of nitroarenes using viologen as an electron phase-transfer catalyst. Tetrahedron Lett. 34, 7445–7446 (1993).
van Dam, H. T. & Ponjee, J. J. Electrochemically generated colored films of insoluble viologen radical compounds. J. Electrochem. Soc. 121, 1555–1558 (1974).
Bruinink, J. & Kregting, C. G. A. Modified viologens with improved electrochemical properties for display applications. J. Electrochem. Soc. 124, 1854–1858 (1977).
Bruinink, J. & Kregting, C. G. A. The voltammetric behavior of some viologens at SnO2 electrodes. J. Electrochem. Soc. 125, 1397–1401 (1978).
Yasuda, A., Mori, H. & Seto, J. Electrochromic properties of alkylviologen-cyclodextrin systems. J. Appl. Electrochem. 17, 567–573 (1987).
Harriman, A. & West, M. A. Photogeneration of Hydrogen (Academic Press, 1982).
Grätzel, M. Energy Resources through Photochemistry and Catalysis (Academic Press, 1983).
Adar, E., Degani, Y., Goren, Z. & Willner, I. Photosensitized electron-transfer reactions in beta-cyclodextrin aqueous media: effects on dissociation of ground-state complexes, charge separation, and hydrogen evolution. J. Am. Chem. Soc. 108, 4696–4700 (1986).
Trabolsi, A. et al. Redox-driven switching in pseudorotaxanes. New J. Chem. 33, 254–263 (2009).
Kosower, E. M. & Cotter, J. L. Stable free radicals. II. The reduction of 1-methyl-4-cyanopyridinium ion to methylviologen cation radical. J. Am. Chem. Soc. 86, 5524–5527 (1964).
Evans, A. G., Evans, J. C. & Baker, M. W. Bipyridyl radical cations. Part I. Electron spin resonance study of the dimerisation equilibrium of morphamquat radical cation in methanol. J. Chem. Soc. Perkin Trans. 2, 1310–1311 (1975).
Evans, A. G., Evans, J. C. & Baker, M. W. Electron spin resonance study of the dimerization equilibrium of the radical cation of 1,1'-diethyl-4,4'-bipyridylium diiodide in methanol. J. Am. Chem. Soc. 99, 5882–5884 (1977).
Claude-Montigny, B., Merlin, A. & Tondre, C. Microenvironment effects on the kinetics of electron-transfer reactions involving dithionite ions and viologens. 2. Stabilization of ion radicals by polyelectrolytes and dimerization kinetics of dialkyl viologens. J. Phys. Chem. 96, 4432–4437 (1992).
Meisel, D., Mulac, W. A. & Metheson, M. S. Catalysis of methyl viologen radical reactions by polymer-stabilized gold sols. J. Phys. Chem. 85, 179–187 (1981).
Furue, M. & Nozakura, S. Photoinduced two-electron reduction of methyl viologen dimer by 2-propanol through intramolecular process an formation of viologen radical cation dimer. Chem. Lett. 9, 821–824 (1980).
Park, J. W., Choi, N. H. & Kim, J. H. Facile dimerization of viologen radical cations covalently bonded to β-cyclodextrin and suppression of the dimerization by β-cyclodextrin and amphiphiles. J. Phys. Chem. 100, 769–774 (1996).
Quintela, P. A. & Kaifer, A. E. Electrochemistry of methylviologen in the presence of sodium decyl sulfate. Langmuir 3, 769–773 (1987).
Ito, M., Sasaki, H. & Takahashi, M. Infrared spectra and dimer structure of reduced viologen compounds. J. Phys. Chem. 91, 3932–3934 (1987).
Meisel, D., Mulac, W. A. & Metheson, M. S. Catalysis of methyl viologen radical reactions by polymer-stabilized gold sols. J. Phys. Chem. 85, 179–187 (1981).
Johnson, C. S. & Gutowsky, H. S. High-resolution ESR spectra of photochemically generated free radicals: the viologens J. Chem. Phys. 39, 58–62 (1963).
Gaudiello, J. G., Ghosh, P. K. & Jones, C. C. Polymer films on electrodes. 17. The application of simultaneous electrochemical and electron spin resonance techniques for the study of two viologen-based chemically modified electrodes J. Am. Chem. Soc. 107, 3027–3032 (1985).
Stoddart, J. F. & Colquhoun, H. M. Big and little Meccano. Tetrahedron 64, 8231–8263 (2008).
Stoddart, J. F. The chemistry of the mechanical bond. Chem. Soc. Rev. 38, 1802–1820 (2009).
Odell, B. et al. Cyclobis (paraquat-p-phenylene). A tetracationic multipurpose receptor. Angew. Chem. Int. Ed. Engl. 27, 1547–1550 (1988).
Asakawa, M. et al. Improved template-directed synthesis of cyclobis(paraquat-p-phenylene). J. Org. Chem. 61, 9591–9595 (1996).
Jeppesen, J. O. et al. Amphiphilic bistable rotaxanes. Chem. Eur. J. 9, 2982–3007 (2003).
Choi, J. W. et al. Ground-state equilibrium thermodynamics and switching kinetics of bistable [2]rotaxanes switched in solution, polymer gels, and molecular electronic devices. Chem. Eur. J. 12, 261–279 (2006).
Liu, Y. et al. Structures and properties of self-assembled monolayers of bistable [2]rotaxanes on Au (111) surfaces from molecular dynamics simulations validated by experiment. J. Am. Chem. Soc. 127, 9745–9759 (2005).
Julury, B. K. et al. A mechanical actuator driven electrochemically by artificial molecular muscles. ACS Nano 3, 291–300 (2009).
Nguyen, T. D. et al. I. Design and optimization of molecular nanovalves based on redox-switchable rotaxanes J. Am. Chem. Soc. 129, 626–634 (2007).
Collier, C. P. et al. A [2]catenane-based solid state electronically reconfigurable switch. Science 289, 1172–1175 (2000).
Green, J. E. et al. A 160-kilobit molecular electronic memory patterned at 1011 bits per square centimetre. Nature 445, 414–417 (2007).
Tseng, H.-R. et al. Redox-controllable amphiphilic [2]rotaxanes. Chem. Eur. J. 10, 155–172 (2004).
Liu, Y., Flood, A. H. & Stoddart, J. F. Thermally and electrochemically controllable self-complexing molecular switches. J. Am. Chem. Soc. 126, 9150–9151 (2004).
Anelli, P.-L., Spencer, N. & Stoddart, J. F. A molecular shuttle. J. Am. Chem. Soc. 113, 5131–5133 (1991).
Coskun, A. et al. A light-gated STOP-GO molecular shuttle. J. Am. Chem. Soc. 131, 2493–2495 (2009).
Ashton, P. R., Belohradsky, M., Phip, D., Spencer, N. & Stoddart, J. F. The self-assembly of [2]- and [3]rotaxanes by slippage. J. Chem. Soc., Chem. Commun. 1269–1274 (1993).
Kosower, E. M. & Hajdu, J. Pyridinyl diradical pi.-mer. Magnesium iodide complexes. J. Am. Chem. Soc. 93, 2534–2535 (1971).
Gueder, W., Hünig, S. & Suchy, A. Single and double bridged viologens and intramolecular pimerization of their cation radicals. Tetrahedron 42, 1665–1677 (1986).
Bard, A. J. & Faulkner, L. R. Electrochemical Methods Fundamentals and Applications (John Wiley & Sons, 1980).
Benitez, D., Tkatchouk, E., Yoon, I., Stoddart, J. F. & Goddard, W. A. III. Experimentally-based recommendations of density functionals for predicting properties in mechanically interlocked molecules. J. Am. Chem. Soc. 130, 14928–14929 (2008).
Truhlar, D. G. Molecular modeling of complex chemical systems. J. Am. Chem. Soc. 130, 16824–16827 (2008).
Reed, A. E., Weinstock, R. B. & Weinhold, F. Natural-population analysis. J. Chem. Phys. 83, 735–746 (1985).
Choi, J. W. et al. Ground-state equilibrium thermodynamics and switching kinetics of bistable [2]rotaxanes switched in solution, polymer gels, and molecular electronic devices. Chem. Eur. J. 12, 261–279 (2006).
Schweiger, A. & Jeschke, G. Principles of Pulse Electron Paramagnetic Resonance (Oxford University Press, 2001).
Zhao, Y.-L., Aprahamian, I., Trabolsi, A., Erina, N. & Stoddart, J. F. Organogel formaton by a cholesterol-stoppered bistable [2]rotaxane and its dumbbell precursor. J. Am. Chem. Soc. 130, 6348–6350 (2008).
Flood, A. H., Nygaard, S., Laursen, B. W., Jeppesen, J. O. & Stoddart, J. F. Locking down the electronic structure of (monopyrrolo)tetrathiafulvalene in [2]rotaxanes. Org. Lett. 8, 2205–2208 (2006).
Han, B., Li, Z., Wandlowski, T., Błaszczyk, A. & Mayor, M. Potential-induced redox switching in viologen self-assembled monolayers: An ATR-SEIRAS approach. J. Phys. Chem. C 111, 13855–13863 (2007).
Aprahamian, I., Olsen, J. C., Trabolsi, A. & Stoddart, J. F. Tetrathiafulvalene radical cation dimerization in a bistable tripodal [4]rotaxane. Chem. Eur. J. 14, 3889–3895 (2008).
Deng, W. Q., Flood, A. H., Stoddart, J. F. & Goddard III, W. A. An electrochemical color-switchable RGB dye: tristable [2]catenane. J. Am. Chem. Soc. 127, 15994–15995 (2005).
Ikeda, T. et al. Toward electrochemically controllable tristable three-station [2]catenanes. Chem. Asian J. 2, 76–93 (2007).
Leigh, D. A., Wong, J. K. Y., Dehez, F. & Zerbetto, F. Unidirectional rotation in a mechanically interlocked molecular rotor. Nature 424, 174–179 (2003).
Acknowledgements
The research was supported by the US Air Force Office of Scientific Research (AFOSR) under their Multidisciplinary University Research Initiative (MURI), award number FA9550-07-1-0534 and by the US National Science Foundation (NSF) under grant numbers CHE-0718928 (M.R.W.) and CHE-0924620 (J.F.S.). M.T.C thanks the Link Foundation for a fellowship. MSC facilities were funded by grants from ARO-DURIP and ONR-DURIP.
Author information
Authors and Affiliations
Contributions
A.T. and J.F.S. conceived the project and prepared the manuscript. A.T., A.C.F., N.M.K., K.C. and M.E.B. synthesized the different molecules studied in this work. A.C.F. was responsible for electrochemical and spectroelectrochemical studies and the determination of the binding energy. D.C.F. performed the NMR spectroscopic studies. J.C.O. performed preliminary force-field simulations. D.B., E.T., and W.A.G.III performed DFT calculations. M.T.C., R.C., and M.R.W. performed all the EPR measurements. H.A.K. provided all the graphical representations used in this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 2005 kb)
Rights and permissions
About this article
Cite this article
Trabolsi, A., Khashab, N., Fahrenbach, A. et al. Radically enhanced molecular recognition. Nature Chem 2, 42–49 (2010). https://doi.org/10.1038/nchem.479
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.479
This article is cited by
-
Analytical noncovalent electrochemistry for battery engineering
Nature Chemical Engineering (2024)
-
An electric molecular motor
Nature (2023)
-
Associative pyridinium electrolytes for air-tolerant redox flow batteries
Nature (2023)
-
Electron-catalysed molecular recognition
Nature (2022)
-
A radical departure for supramolecular chemistry
Nature Reviews Chemistry (2021)