Abstract
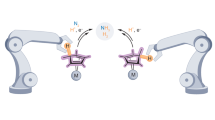
Bond activation at a transition metal centre is a key fundamental step in numerous chemical transformations. The oxidative addition of element–hydrogen bonds, for example, represents a critical step in a range of widely applied catalytic processes. Despite this, experimental studies defining steps along the bond activation pathway are very rare. In this work, we report on fundamental studies defining a double oxidative activation pathway: combined experimental and computational approaches yield structural snapshots of the simultaneous activation of both bonds of a β-diketiminate-stabilized GaH2 unit at a single metal centre. Systematic variation of the supporting phosphine ligands and single crystal X-ray/neutron diffraction are exploited in tandem to allow structural visualization of the activation process, from a η2-H,H σ-complex showing little Ga–H bond activation, through species of intermediate geometry featuring stretched Ga–H and compressed M–H/M–Ga bonds, to a fully activated metal dihydride featuring a neutral (carbene-type) N-heterocyclic GaI ligand.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
18 December 2017
References 23–27 and 31–38 in the introductory paragraphs describe literature precedent for the activation of two E–H bonds at a transition metal centre for E = B or C). It should be noted that experimental precedent also exists for the analogous activation of Si–H bonds and the manuscript has been modified to include additional citation of this chemistry (refs 22, 28–30). References 22. Lipke, M. C., Liberman-Martin, A. L. & Tilley, T. D. Electrophilic activation of silicon–hydrogen bonds in catalytic hydrosilations. Angew. Chem. Int. Ed. 56, 2260–2294 (2017). 28. Thomas, C. M. & Peters, J. C. An η3-H2SiR2 adduct of [{PhB(CH2PiP2)3}FeH. Angew. Chem. Int. Ed. 45, 776–780 (2006). 29. Faluso, M. E., Glaser, P. B. & Tilley, T. D. Cp*;(PiPr3)RuOTf: a reagent for access to ruthenium silylene complexes. Organometallics 30, 5524–5531 (2011). 30. Faluso, M. E., Lipke, M. C. & Tilley, T. D. Structural and mechanistic investigation of a cationic hydrogen-substituted ruthenium silylene catalyst for alkene hydrosilation. Chem. Sci. 4, 3882–3887 (2013).
References
Hartwig, J. F. Organotransition Metal Chemistry: from Bonding to Catalysis (University Science Books, 2010).
Kubas, G. J. Metal Dihydrogen and σ-Bond Complexes: Structure, Theory and Reactivity (Kluwer Academic/Plenum Publishers, 2001).
Crabtree, R. H. The organometallic chemistry of alkanes. Chem. Rev. 85, 245–269 (1985).
Arndtsen, B. A., Bergman, R. G., Mobley, T. A. & Peterson, T. H. Selective intermolecular carbon–hydrogen bond activation by synthetic metal complexes in homogeneous solution. Acc. Chem. Res. 28, 154–162 (1995).
Shilov, A. E. & Shul'pin, G. B. Activation of C–H bonds by metal complexes. Chem. Rev. 97, 2879–2932 (1997).
Jones, W. D. Isotope effects in C–H bond activation reactions by transition metals. Acc. Chem. Res. 36, 140–146 (2003).
Labinger, J. A. & Bercaw, J. E. Understanding and exploiting C–H activation. Nature 417, 507–514 (2002).
Crabtree, R. H. Organometallic C–H activation. J. Organomet. Chem. 689, 4083–4091 (2004).
Schubert, U. Coordination of Si–H σ bonds to transition metals. Adv. Organomet. Chem. 30, 151–187 (1990).
Alcaraz, G. & Sabo-Etienne, S. Coordination and dehydrogenation of amine-boranes at metal centers. Angew. Chem. Int. Ed. 49, 7170–7179 (2010).
Riddlestone, I. M., Abdalla, J. A. B. & Aldridge, S. Coordination and activation of E–H bonds (E = B, Al, Ga) Adv. Organomet. Chem. 63, 1–38 (2015).
Kubas, G. J., Ryan, R. R., Swanson, B. I., Vergamini, P. J. & Wasserman, H. J. Characterization of the first examples of isolable molecular hydrogen complexes, M(CO)3(PR3)2(H2) (M = molybdenum or tungsten; R = Cy or isopropyl). evidence for a side-on bonded dihydrogen ligand. J. Am. Chem. Soc. 106, 451–452 (1984).
Hall, C. & Perutz, R. N. Transition metal alkane complexes. Chem. Rev. 96, 3125–3146 (1996).
Cowan, A. J. & George, M. W. Formation and reactivity of organometallic alkane complexes. Coord. Chem. Rev. 252, 2504–2511 (2008).
Bernskoetter, W. H., Schauer, C. K., Goldberg, K. I. & Brookhart, M. Characterization of a rhodium(I) σ-methane complex in solution. Science 326, 553–556 (2009).
Pike, S. D. et al. Synthesis and characterization of a rhodium(I) σ-alkane complex in the solid state. Science 337, 1648–1651 (2012).
Pandey, K. K. Transition metal σ-borane complexes. Coord. Chem. Rev. 253, 37–55 (2009).
Lin, Z. Transition metal σ-borane complexes. Struct. Bonding (Berlin) 130, 123–148 (2008).
Crabtree, R. H., Holt, E. M., Lavin, M. & Morehouse, S. M. Inter- vs. intramolecular carbon-hydrogen activation: a carbon–hydrogen–iridium bridge in [IrH2(8-methylquinoline)L2]BF4 and a CH + M → CMH reaction trajectory. Inorg. Chem. 24, 1986–1992 (1985).
Ekkert, O., White, A. J. P. & Crimmin, M. Trajectory of approach of a zinc–hydrogen bond to transition metals. Angew. Chem. Int. Ed. 55, 16031–16034 (2016).
Green, J. C., Green, M. L. H. & Parkin, G. The occurrence and representation of three-centre two-electron bonds in covalent inorganic compounds. Chem. Commun. 48, 11481–11503 (2012).
Lipke, M. C., Liberman-Martin, A. L & Tilley, T. D. Electrophilic activation of silicon–hydrogen bonds in catalytic hydrosilations. Angew. Chem. Int. Ed. 56, 2260–2294 (2017).
Alcaraz, G., Helmstedt, U., Clot, E., Vendier, L. & Sabo-Etienne, S. A terminal borylene ruthenium complex from B−H activation to reversible hydrogen release. J. Am. Chem. Soc. 130, 12878–12879 (2008).
Alcaraz, G., Grellier, M. & Sabo-Etienne, S. Bis σ-bond dihydrogen and borane ruthenium complexes: bonding nature, catalytic applications, and reversible hydrogen release. Acc. Chem. Res. 42, 1640–1649 (2009).
Turner, J. et al. Formation of sub-valent carbenoid ligands by metal-mediated dehydrogenation chemistry: coordination and activation of H2Ga{(NDippCMe)2CH}. Chem. Sci. 4, 4245–4250 (2013).
Abdalla, J. A. B. et al. Coordination and activation of Al-H and Ga-H bonds. Chem. Eur. J. 20, 17624–17634 (2014).
O'Neill, M. et al. Borane to boryl hydride to borylene dihydride: explicit demonstration of boron-to-metal α-hydride migration in aminoborane activation. J. Am. Chem. Soc. 133, 11500–11503 (2012).
Thomas, C. M. & Peters, J. C. An η3-H2SiR2 adduct of [{PhB(CH2PiPr2)3}FeH. Angew. Chem. Int. Ed. 45, 776–780 (2006).
Faluso, M. E., Glaser, P. B. & Tilley, T. D. Cp*(PiPr3)RuOTf: a reagent for access to ruthenium silylene complexes. Organometallics. 30, 5524–5531 (2011).
Faluso, M. E., Lipke, M. C. & Tilley, T. D. Structural and mechanistic investigation of a cationic hydrogen-substituted ruthenium silylene catalyst for alkene hydrosilation. Chem. Sci. 4, 3882–3887 (2013).
Empsall, H. D. et al. Synthesis and X-ray structure of an unusual iridium ylide or carbene complex. Chem. Commun. 589–590 (1977).
Boutry, O. et al. Double C-H activation at the α-carbon of cyclic ethers by Tp*Ir(C2H4)2 . J. Am. Chem. Soc. 114, 7288–7290 (1992).
Li, Z.-W. & Taube, H. Dihydrogen complexes of tetraammineosmium(II) with carbene as co-ligand: facile hydrogen transfer from cyclic ethers to the metal center. J. Am. Chem. Soc. 116, 11584–11585 (1994).
Luecke, H. F., Arndtsen, B. A., Burger, P. & Bergman, R. G. Synthesis of Fischer carbene complexes of iridium by C-H bond activation of methyl and cyclic ethers: evidence for reversible α-hydrogen migration. J. Am. Chem. Soc. 118, 2517–2518 (1996).
Holtcamp, M. W., Labinger, J. A. & Bercaw, J. E. C-H activation at cationic platinum(II) centers. J. Am. Chem. Soc. 119, 848–849 (1997).
Coalter, J. N., Ferrando, G. & Caulton, K. G. Geminal dehydrogenation of a C(sp3) CH2 group by unsaturated Ru(II) or Os(II). New J. Chem. 24, 835–836 (2000).
Lee, D.-H., Chen, J., Faller, J. W. & Crabtree, R. H. Reversible α-elimination in the conversion of N-CH3 to N-CH=Ir by double C-H activation. Chem. Commun. 213–214 (2001).
Whited, M. T. & Grubbs, R. H. Late metal carbene complexes generated by multiple C-H activations: examining the continuum of M=C bond reactivity. Acc. Chem. Res. 42, 1607–1616 (2009).
Bourissou, D., Guerret, O., Gabbaï, F. P. & Bertrand, G. Stable carbenes. Chem. Rev. 100, 39–92 (2000).
Staubitz, A., Robertson, A. P. M. & Manners, I. Ammonia-borane and related compounds as dihydrogen sources. Chem. Rev. 110, 4079–4124 (2010).
Singh, S. et al. Syntheses, characterization, and X-ray crystal structures of β-diketiminate group 13 hydrides, chlorides, and fluorides. Inorg. Chem. 45, 1853–1860 (2006).
Dallanegra, R., Robertson, A. P. M., Chaplin, A. B., Manners, I. & Weller, A. S. Tuning the [L2Rh⋯H3B·NR3]+ interaction using phosphine bite angle. Demonstration by the catalytic formation of polyaminoboranes. Chem. Commun. 47, 3763–3765 (2011).
Cooper, R. I., Thompson, A. L. & Watkin, D. J. CRYSTALS enhancements: dealing with hydrogen atoms in fefinement. J. Appl. Cryst. 43, 1100–1107 (2010).
Cordero, B. et al. Covalent radii revisited. Dalton Trans. 40, 2832–2838 (2008).
Cadenbach, T. et al. Molecular alloys, linking organometallics with intermetallic hume–Rothery phases: the highly coordinated transition metal compounds [M(ZnR)n] (n ≥ 8) containing organo–zinc ligands. J. Am. Chem. Soc. 131, 16063–16077 (2009).
Cadenbach, T., Gemel, C., Zacher, D. & Fischer, R. A. Methylgallium as a terminal ligand in [(Cp*Ga)4Rh(GaCH3)]+. Angew. Chem. Int. Ed. 47, 3438–3441 (2008).
Bollermann, T. et al. Homoleptic hexa and penta gallylene coordinated complexes of molybdenum and rhodium. Inorg. Chem. 50, 5808–5815 (2011).
Freixa, Z. & van Leeuwen, P. Bite angle effects in diphosphine metal catalysts: steric or electronic? Dalton Trans. 77, 1890–1901 (2003).
DuBois, D. L. et al. Hydride transfer from rhodium complexes to triethylborane. Organometallics 25, 4414–4419 (2006).
Wilson, A. D., Miller, A. J. M., DuBois, D. L., Labinger, J. A. & Bercaw, J. E. Thermodynamic studies of [H2Rh(diphosphine)2]+ and [HRh(diphosphine)2(CH3CN)]2+ complexes in acetonitrile. Inorg. Chem. 49, 3918–3926 (2010).
Sewell, L. J., Lloyd-Jones, G. C. & Weller, A. S. Development of a generic mechanism for the dehydrocoupling of amine-boranes: a stoichiometric, catalytic, and kinetic study of H3B·NMe2H using the [Rh(PCy3)2]+ fragment. J. Am. Chem. Soc. 134, 3598–3610 (2012).
Brookhart, M., Green, M. L. H. & Parkin, G. Agostic interactions in transition metal compounds. Proc. Natl Acad. Sci. USA 104, 6908–6914 (2007).
Smart, K. A. et al. Step by step introduction of silazane moieties at ruthenium different extents of Ru–H–Si bond activation. Inorg. Chem. 52, 2654–2661 (2013).
Smart, K. A. et al. Nature of Si-H interactions in a series of ruthenium silazane complexes using multinuclear solid-state NMR and neutron diffraction. Inorg. Chem. 53, 1156–1165 (2014).
Edwards, A. J. Neutron diffraction — recent applications to chemical structure determination. Aus. J. Chem. 64, 869–872 (2011).
Aldridge, S. & Downs, A. J. Hydrides of the main group metals; new variations on an old theme. Chem. Rev. 101, 3305–3365 (2001).
Bau, R. & Drabnis, M. H. Structures of transition metal hydrides determined by neutron diffraction. Inorg. Chim. Acta 259, 27–50 (1997).
Bader, R. F. W. A quantum theory of molecular structure and its applications. Chem. Rev. 91, 893–928 (1991).
Mitoraj, M. P., Michalak, A. & Ziegler, T. A combined charge and energy decomposition scheme for bond analysis. J. Chem. Theory Comput. 5, 962–975 (2009).
Acknowledgements
This work was supported by the EPSRC (studentship to J.A.B.A.); ANSTO is thanked for the allocation of discretionary neutron beam-time on KOALA to proposal DB3706 and a further allocation to proposal P3932. C.S. thanks the Alexander von Humboldt Stiftung for a Feodor Lynen Fellowship.
Author information
Authors and Affiliations
Contributions
J.A.B.A. and A.C. synthesized and characterized the compounds. R.T., A.J.E. and A.L.T. collected the single-crystal X-ray and neutron crystallographic data and determined the crystal structures. J.A.B.A. and C.P.S. carried out the DFT calculations. S.A. generated and managed the project and wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 10840 kb)
Supplementary information
Crystallographic data for compound 3 (CIF 1160 kb)
Rights and permissions
About this article
Cite this article
Abdalla, J., Caise, A., Sindlinger, C. et al. Structural snapshots of concerted double E–H bond activation at a transition metal centre. Nature Chem 9, 1256–1262 (2017). https://doi.org/10.1038/nchem.2792
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2792
This article is cited by
-
A hexagonal planar transition-metal complex
Nature (2019)