Abstract
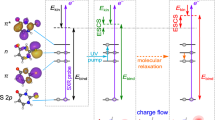
Photoionization is at the heart of X-ray photoelectron spectroscopy (XPS), which gives access to important information on a sample's local chemical environment. Local and non-local electronic decay after photoionization—in which the refilling of core holes results in electron emission from either the initially ionized species or a neighbour, respectively—have been well studied. However, electron-transfer-mediated decay (ETMD), which involves the refilling of a core hole by an electron from a neighbouring species, has not yet been observed in condensed phase. Here we report the experimental observation of ETMD in an aqueous LiCl solution by detecting characteristic secondary low-energy electrons using liquid-microjet soft XPS. Experimental results are interpreted using molecular dynamics and high-level ab initio calculations. We show that both solvent molecules and counterions participate in the ETMD processes, and different ion associations have distinctive spectral fingerprints. Furthermore, ETMD spectra are sensitive to coordination numbers, ion–solvent distances and solvent arrangement.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Howell, R. W. Auger processes in the 21st century. Int. J. Radiat. Biol. 84, 959–975 (2008).
Petit, M. et al. X-ray photolysis to release ligands from caged reagents by an intramolecular antenna sensitive to magnetic resonance imaging. Angew. Chem. Int. Ed. 50, 9708–9711 (2011).
Cederbaum, L. S., Zobeley, J. & Tarantelli, F. Giant intermolecular decay and fragmentation of clusters. Phys. Rev. Lett. 79, 4778–4781 (1997).
Averbukh, V. et al. Interatomic electronic decay processes in singly and multiply ionized clusters. J. Electron Spectrosc. Relat. Phenom. 183, 36–47 (2011).
Hergenhahn, U. Production of low kinetic energy electrons and energetic ion pairs by intermolecular Coulombic decay. Int. J. Radiat. Biol. 88, 871–883 (2012).
Aziz, E. F., Ottosson, N., Faubel, M., Hertel, I. V. & Winter, B. Interaction between liquid water and hydroxide revealed by core-hole de-excitation. Nature 455, 89–91 (2008).
Pokapanich, W. et al. Ionic-charge dependence of the intermolecular Coulombic decay time scale for aqueous ions probed by the core-hole clock. J. Am. Chem. Soc. 133, 13430–13436 (2011).
Slavíček, P., Winter, B., Cederbaum, L. S. & Kryzhevoi, N. V. Proton-transfer mediated enhancement of nonlocal electronic relaxation processes in X-ray irradiated liquid water. J. Am. Chem. Soc. 136, 18170–18176 (2014).
Jahnke, T. et al. Ultrafast energy transfer between water molecules. Nat. Phys. 6, 139–142 (2010).
Mucke, M. et al. A hitherto unrecognized source of low-energy electrons in water. Nat. Phys. 6, 143–146 (2010).
Zobeley, J., Santra, R. & Cederbaum, L. S. Electronic decay in weakly bound heteroclusters: energy transfer versus electron transfer. J. Chem. Phys. 115, 5076–5088 (2001).
Stumpf, V., Kryzhevoi, N. V., Gokhberg, K. & Cederbaum, L. S. Enhanced one-photon double ionization of atoms and molecules in an environment of different species. Phys. Rev. Lett. 112, 193001 (2014).
LaForge, A. C. et al. Enhanced ionization of embedded clusters by electron-transfer-mediated decay in helium nanodroplets. Phys. Rev. Lett. 116, 203001 (2016).
Förstel, M., Mucke, M., Arion, T., Bradshaw, A. M. & Hergenhahn, U. Autoionization mediated by electron transfer. Phys. Rev. Lett. 106, 33402 (2011).
Sakai, K. et al. Electron-transfer-mediated decay and interatomic Coulombic decay from the triply ionized states in argon dimers. Phys. Rev. Lett. 106, 33401 (2011).
Yan, S. et al. Observation of interatomic Coulombic decay and electron-transfer- mediated decay in high-energy electron-impact ionization of Ar2 . Phys. Rev. A 88, 42712 (2013).
Pernpointner, M., Kryzhevoi, N. V. & Urbaczek, S. Possible electronic decay channels in the ionization spectra of small clusters composed of Ar and Kr: a four-component relativistic treatment. J. Chem. Phys. 129, 24304 (2008).
Stumpf, V., Kolorenč, P., Gokhberg, K. & Cederbaum, L. S. Efficient pathway to neutralization of multiply charged ions produced in Auger processes. Phys. Rev. Lett. 110, 258302 (2013).
Müller, I. B. & Cederbaum, L. S. Electronic decay following ionization of aqueous Li+ microsolvation clusters. J. Chem. Phys. 122, 94305 (2005).
Stumpf, V., Gokhberg, K. & Cederbaum, L. S. The role of metal ions in X-ray-induced photochemistry. Nat. Chem. 8, 237–241 (2016).
Weber, R. et al. Photoemission from aqueous alkali-metal-iodide salt solutions using EUV synchrotron radiation. J. Phys. Chem. B 108, 4729–4736 (2004).
Bouazizi, S. & Nasr, S. Concentration effects on aqueous lithium chloride solutions. Molecular dynamics simulations and X-ray scattering studies. J. Mol. Liq. 197, 77–83 (2014).
Petit, L., Vuilleumier, R., Maldivi, P. & Adamo, C. Ab initio molecular dynamics study of a highly concentrated LiCl aqueous solution. J. Chem. Theory Comput. 4, 1040–1048 (2008).
Harsanyi, I. & Pusztai, L. Hydration structure in concentrated aqueous lithium chloride solutions: a reverse Monte Carlo based combination of molecular dynamics simulations and diffraction data. J. Chem. Phys. 137, 204503 (2012).
Pluhařová, E., Fischer, H. E., Mason, P. E. & Jungwirth, P. Hydration of the chloride ion in concentrated aqueous solutions using neutron scattering and molecular dynamics. Mol. Phys. 112, 1230–1240 (2014).
Pluhařová, E., Mason, P. E. & Jungwirth, P. Ion pairing in aqueous lithium salt solutions with monovalent and divalent counter-anions. J. Phys. Chem. A 117, 11766–11773 (2013).
Aragones, J. L., Rovere, M., Vega, C. & Gallo, P. Computer simulation study of the structure of LiCl aqueous solutions: test of non-standard mixing rules in the ion interaction. J. Phys. Chem. B 118, 7680–7691 (2014).
Xu, J.-J., Yi, H.-B., Li, H.-J. & Chen, Y. Ionic solvation and association in LiCl aqueous solution: a density functional theory, polarised continuum model and molecular dynamics investigation. Mol. Phys. 112, 1710–1723 (2014).
Öhrwall, G. et al.. Charge dependence of solvent-mediated intermolecular Coster−Kronig decay dynamics of aqueous ions. J. Phys. Chem. B 114, 17057–17061 (2010).
Hefter, G . When spectroscopy fails: the measurement of ion pairing. Pure Appl. Chem. 78, 1571–1586 (2006).
van der Vegt, N. F. A. et al. Water-mediated ion pairing: occurrence and relevance. Chem. Rev. 116, 7626–7641 (2016).
Näslund, L.-Å. et al. Direct evidence of orbital mixing between water and solvated transition-metal ions: an oxygen 1s XAS and DFT study of aqueous systems. J. Phys. Chem. A 107, 6869–6876 (2003).
Jiang, B. et al. The anion effect on Li+ ion coordination structure in ethylene carbonate solutions. J. Phys. Chem. Lett. 7, 3554–3559 (2016).
Seidel, R., Thürmer, S. & Winter, B. Photoelectron spectroscopy meets aqueous solution: studies from a vacuum liquid microjet. J. Phys. Chem. Lett. 2, 633–641 (2011).
Berendsen, H., Grigera, J. & Straatsma, T. The missing term in effective pair potentials. J. Phys. Chem. 91, 6269–6271 (1987).
Leontyev, I. & Stuchebrukhov, A. Accounting for electronic polarization in non- polarizable force fields. Phys. Chem. Chem. Phys. 13, 2613–2626 (2011).
Bussi, G., Donadio, D. & Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 126, 14101 (2007).
Essmann, U. et al.. A smooth particle mesh Ewald method. J. Chem. Phys. 103, 8577–8593 (1995).
Hess, B., Kutzner, C., van der Spoel, D. & Lindahl, E. GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 4, 435–447 (2008).
Schirmer, J. & Barth, A. Higher-order approximations for the particle-particle propagator. Z. Phys. A 317, 267–279 (1984).
Velkov, Y., Miteva, T., Sisourat, N. & Schirmer, J. Intermediate state representation approach to physical properties of dicationic states. J. Chem. Phys. 135, 154113 (2011).
Dunning, T. H. Jr Gaussian basis functions for use in molecular calculations. I. Contraction of (9s5p) atomic basis sets for the first-row atoms. J. Chem. Phys. 53, 2823–2833 (1970).
Kendall, R., Dunning, T. & Harrison, R. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 96, 6796–6806 (1992).
Tarantelli, F., Sgamellotti, A. & Cederbaum, L. S. Many dicationic states and two-hole population analysis as a bridge to Auger spectra: strong localization phenomena in BF3 . J. Chem. Phys. 94, 523–532 (1991).
Acknowledgements
E.M. and P.S. thank Czech Science Foundation for support (project number 13-34168S). N.V.K. and L.S.C. gratefully acknowledge financial support from the Deutsche Forschungsgemeinschaft. L.S.C also gratefully acknowledges funding from the European Research Council (Advanced Investigator Grant no. 692657). I.U., M.N.P. and B.W. gratefully acknowledge support from the Deutsche Forschungsgemeinschaft (DFG Research Unit FOR 1789). The authors thank the BESSY II staff for assistance during the beamtimes. We thank E. Pluhařová for valuable discussions.
Author information
Authors and Affiliations
Contributions
I.U., R.S., S.T., M.N.P. and B.W. conceived, designed and performed the experiments, and analysed the experimental data. E.M. and P.S. performed and analysed the molecular dynamics simulations. N.V.K. computed the theoretical ETMD spectra and analysed them. B.W., P.S. and N.V.K. co-wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 980 kb)
Rights and permissions
About this article
Cite this article
Unger, I., Seidel, R., Thürmer, S. et al. Observation of electron-transfer-mediated decay in aqueous solution. Nature Chem 9, 708–714 (2017). https://doi.org/10.1038/nchem.2727
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2727
This article is cited by
-
Radiation damage by extensive local water ionization from two-step electron-transfer-mediated decay of solvated ions
Nature Chemistry (2023)
-
Triple ionization and fragmentation of benzene trimers following ultrafast intermolecular Coulombic decay
Nature Communications (2022)
-
Attosecond spectroscopy of size-resolved water clusters
Nature (2022)
-
Heavy N+ ion transfer in doubly charged N2Ar van der Waals cluster
Nature Communications (2020)
-
Water acting as a catalyst for electron-driven molecular break-up of tetrahydrofuran
Nature Communications (2020)