Abstract
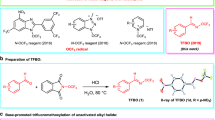
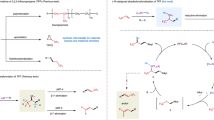
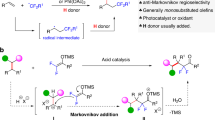
Fluorinated organic compounds are becoming increasingly important in pharmaceuticals, agrochemicals and materials science. The introduction of trifluoromethoxy groups into new drugs and agrochemicals has attracted much attention due to their strongly electron-withdrawing nature and high lipophilicity. However, synthesis of trifluoromethoxylated organic molecules is difficult owing to the decomposition of trifluoromethoxide anion and β-fluoride elimination from transition-metal–trifluoromethoxide complexes, and no catalytic enantioselective trifluoromethoxylation reaction has been reported until now. Here, we present an example of an asymmetric silver-catalysed intermolecular bromotrifluoromethoxylation of alkenes with trifluoromethyl arylsulfonate (TFMS) as a new trifluoromethoxylation reagent. Compared to other trifluoromethoxylation reagents, TFMS is easily prepared and thermally stable with good reactivity. In addition, this reaction is operationally simple, scalable and proceeds under mild reaction conditions. Furthermore, broad scope and good functional group compatibility has been demonstrated by application of the method to the bromotrifluoromethoxylation of double bonds in natural products and natural product derivatives.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Müller, K., Faeh, C. & Diederich, F. Fluorine in pharmaceuticals: looking beyond intuition. Science 317, 1881–1886 (2007).
Berger, R., Resnati, G., Metrangolo, P., Weber, E. & Hulliger, J. Organic fluorine compounds: a great opportunity for enhanced materials properties. Chem. Soc. Rev. 40, 3496–3508 (2011).
Isanbor, C. & O'Hagan, D. Fluorine in medicinal chemistry: a review of anti-cancer agents. J. Fluorine Chem. 127, 303–319 (2006).
Purser, S., Moore, P. R., Swallow, S. & Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 37, 320–330 (2008).
Jeschke, P. The unique role of fluorine in the design of active ingredients for modern crop protection. ChemBioChem. 5, 570–589 (2004).
Shimizu, M. & Hiyama, T. Modern synthetic methods for fluorine-substituted target molecules. Angew. Chem. Int. Ed. 44, 214–231 (2005).
Leroux, F., Jeschke, P. & Schlosser, M. α-Fluorinated ethers, thioethers, and amines: anomerically biased species. Chem. Rev. 105, 827–856 (2005).
Jeschke, P., Baston, E . & Leroux, F. R. α-Fluorinated ethers as “exotic” entity in medicinal chemistry. Mini-Rev. Med. Chem. 7, 1027–1034 (2007).
Manteau, B., Pazenok, S., Vors, J. P. & Leroux, F. R. New trends in the chemistry of α-fluorinated ethers, thioethers, amines and phosphines. J. Fluorine Chem. 131, 140–158 (2010).
Landelle, G., Panossian, A. & Leroux, F. R. Trifluoromethyl ethers and -thioethers as tools for medicinal chemistry and drug discovery. Curr. Top. Med. Chem. 14, 941–951 (2014).
Leroux, F., Manteau, B., Vors, J. & Pazenok, S. Trifluoromethyl ethers – synthesis and properties of an unusual substituent. Beilstein J. Org. Chem. 4, 13 (2008).
Liang, T., Neumann, C. N. & Ritter, T. Introduction of fluorine and fluorine-containing functional groups. Angew. Chem. Int. Ed. 52, 8214–8264 (2013).
Tlili, A., Toulgoat, F. & Billard, T. Synthetic approaches to trifluoromethoxy-substituted compounds. Angew. Chem. Int. Ed. 55, 11726–11735 (2016).
Yagupol'skii, L. M. Synthesis of derivatives of phenyl trifluoromethyl ether. Dokl. Akad. Nauk SSSR 105, 100–102 (1955).
Sheppard, W. A. α-Fluorinated ethers. I. aryl fluoroalkyl ethers. J. Org. Chem. 29, 1–11 (1964).
Kuroboshi, M., Suzuki, K. & Hiyama, T. Oxidative desulfurization-fluorination of xanthates. A convenient synthesis of trifluoromethyl ethers and difluoro (methylthio) methyl ethers. Tetrahedron Lett. 33, 4173–4176 (1992).
Umemoto, T. Electrophilic perfluoroalkylating agents. Chem. Rev. 96, 1757–1778 (1996).
Umemoto, T., Adachi, K. & Ishihara, S. CF3 oxonium salts, O-(trifluoromethyl) dibenzofuranium salts: in situ synthesis, properties, and application as a real CF3+ species reagent. J. Org. Chem. 72, 6905–6917 (2007).
Stanek, K., Koller, R. & Togni, A. Reactivity of a 10-I-3 hypervalent iodine trifluoromethylation reagent with phenols. J. Org. Chem. 73, 7678–7685 (2008).
Fantasia, S., Welch, J. M. & Togni, A. Reactivity of a hypervalent iodine trifluoromethylating reagent toward THF: ring opening and formation of trifluoromethyl ethers. J. Org. Chem. 75, 1779–1782 (2010).
Koller, R. et al. Zinc-mediated formation of trifluoromethyl ethers from alcohols and hypervalent iodine trifluoromethylation reagents. Angew. Chem. Int. Ed. 48, 4332–4336 (2009).
Liang, A. et al. Regioselective synthesis of N-heteroaromatic trifluoromethoxy compounds by direct O−CF3 bond formation. Chem. Eur. J. 22, 5102–5106 (2016).
Brantley, J. N., Samant, A. V. & Toste, F. D. Isolation and reactivity of trifluoromethyl iodonium salts. ACS Cent. Sci. 2, 341–350 (2016).
Hojczyk, K. N., Feng, P., Zhan, C. & Ngai, M. Y. Trifluoromethoxylation of arenes: synthesis of ortho-trifluoromethoxylated aniline derivatives by OCF3 migration. Angew. Chem. Int. Ed. 53, 14559–14563 (2014).
Feng, P., Lee, K. N., Lee, J. W., Zhan, C. & Ngai, M. Y. Access to a new class of synthetic building blocks via trifluoromethoxylation of pyridines and pyrimidines. Chem. Sci. 7, 424–429 (2016).
Lee, K. N., Lei, Z., Morales-Rivera, C. A., Liu, P. & Ngai, M. Y. Mechanistic studies on intramolecular C–H trifluoromethoxylation of (hetero) arenes via OCF3-migration. Org. Biomol. Chem. 14, 5599–5605 (2016).
Huang, C., Liang, T., Harada, S., Lee, E. & Ritter, T. Silver-mediated trifluoromethoxylation of aryl stannanes and arylboronic acids. J. Am. Chem. Soc. 133, 13308–13310 (2011).
Liu, J. et al. Silver-mediated oxidative trifluoromethylation of phenols: direct synthesis of aryl trifluoromethyl ethers. Angew. Chem. Int. Ed. 54, 11839–11842 (2015).
Liu, J., Xu, X. & Qing, F. Silver-mediated oxidative trifluoromethylation of alcohols to alkyl trifluoromethyl ethers. Org. Lett. 17, 5048–5051 (2015).
Chen, C., Chen, P. & Liu, G. Palladium-catalyzed intramolecular aminotrifluoromethoxylation of alkenes. J. Am. Chem. Soc. 137, 15648–15651 (2015).
Zha, G. et al. Silver-mediated direct trifluoromethoxylation of α-diazo esters via the –OCF3 anion. Chem. Commun. 52, 7458–7461 (2016).
Zhang, Q. et al. Fluorodecarboxylation for the synthesis of trifluoromethyl aryl ethers. Angew. Chem. Int. Ed. 55, 9758–9762 (2016).
Zhang, C. & Vicic, D. A. Oxygen-bound trifluoromethoxide complexes of copper and gold. Organometallics 31, 7812–7815 (2012).
Chen, S. et al. Aryl-BIAN-ligated silver (I) trifluoromethoxide complex. Dalton Trans. 44, 19682–19686 (2015).
Rozen, S. Selective fluorinations by reagents containing the OF group. Chem. Rev. 96, 1717–1736 (1996).
Noftle, R. E. & Cady, G. H. Preparation and properties of bis (trifluoromethylsulfuryl) peroxide and trifluoromethyl trifluoromethanesulfonate. Inorg. Chem. 4, 1010–1012 (1965).
Taylor, S. L. & Martin, J. C. Trifluoromethyl triflate: synthesis and reactions. J. Org. Chem. 52, 4147–4156 (1987).
Kolomeitsev, A. A., Vorobyev, M. & Gillandt, H. Versatile application of trifluoromethyl triflate. Tetrahedron Lett. 49, 449–454 (2008).
Marrec, O., Billard, T., Vors, J., Pazenok, S. & Langlois, B. R. A new and direct trifluoromethoxylation of aliphatic substrates with 2, 4-dinitro (trifluoromethoxy) benzene. Adv. Synth. Catal. 352, 2831–2837 (2010).
Koller, R., Huchet, Q., Battaglia, P., Welch, J. M. & Togni, A . Acid-mediated formation of trifluoromethyl sulfonates from sulfonic acids and a hypervalent iodine trifluoromethylating agent. Chem. Commun. 2009, 5993–5995 (2009).
Sokolenko, T. M., Davydova, Y. A. & Yagupolskii, Y. L. Efficient synthesis of 5′-fluoroalkoxythiazoles via α-bromo-α-fluoroalkoxyacetophenones Hantzsch type cyclization with thioureas or thioamides. J. Fluorine Chem. 136, 20–25 (2012).
Acknowledgements
The authors dedicate this manuscript to Qi-lin Zhou on the occasion of his sixtieth birthday. We are grateful for financial support from the State Key Laboratory of Elemento-Organic Chemistry, the National Key Research and Development Program of China (2016YFA0602900) and NFSC (21402098, 21421062, 21522205).
Author information
Authors and Affiliations
Contributions
S.G., F.C., R.G. and L.W. performed the experiments and analysed the data. P.T. designed and directed the project. P.T. wrote the manuscript. All of the authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 8374 kb)
Supplementary information
Crystallographic data for compound 3i. (CIF 213 kb)
Supplementary information
Crystallographic data for compound 3t. (CIF 661 kb)
Supplementary information
Crystallographic data for compound 3x. (CIF 394 kb)
Supplementary information
Crystallographic data for compound 5a. (CIF 256 kb)
Supplementary information
Crystallographic data for compound 5b. (CIF 643 kb)
Rights and permissions
About this article
Cite this article
Guo, S., Cong, F., Guo, R. et al. Asymmetric silver-catalysed intermolecular bromotrifluoromethoxylation of alkenes with a new trifluoromethoxylation reagent. Nature Chem 9, 546–551 (2017). https://doi.org/10.1038/nchem.2711
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2711
This article is cited by
-
Fluorocarbonylation via palladium/phosphine synergistic catalysis
Nature Communications (2023)
-
Synthetic exploration of sulfinyl radicals using sulfinyl sulfones
Nature Communications (2021)
-
Chemoselective desulfurization-fluorination/bromination of carbonofluoridothioates for the O-trifluoromethylation and O-bromodifluoromethylation of alcohols
Science China Chemistry (2021)
-
Selective C-H trifluoromethoxylation of (hetero)arenes as limiting reagent
Nature Communications (2020)
-
Nucleophilic trifluoromethoxylation of alkyl halides without silver
Nature Communications (2020)