Abstract
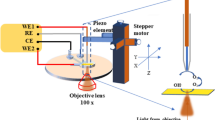
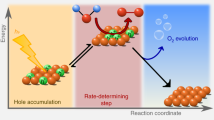
Semiconductor electrodes capable of using solar photons to drive water-splitting reactions, such as haematite (α-Fe2O3), have been the subject of tremendous interest over recent decades. The surface has been found to play a significant role in determining the efficiency of water oxidation with haematite; however, previous works have only allowed hypotheses to be formulated regarding the identity of relevant surface species. Here we investigate the water-oxidation reaction on haematite using infrared spectroscopy under photoelectrochemical (PEC) water-oxidation conditions. A potential- and light-dependent absorption peak at 898 cm−1 is assigned to a FeIV=O group, which is an intermediate in the PEC water-oxidation reaction. These results provide direct evidence of high-valent iron–oxo intermediates as the product of the first hole-transfer reaction on the haematite surface and represent an important step in establishing the mechanism of PEC water oxidation on semiconductor electrodes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kennedy, J. H. & Frese, K. W. Photooxidation of water at α-Fe2O3 electrodes. J. Electrochem. Soc. 125, 709–714 (1978).
Lindgren, T. et al. Aqueous photoelectrochemistry of hematite nanorod array. Sol. Energy Mater. Sol. Cells 71, 231–243 (2002).
Bjorksten, U., Moser, J. & Gratzel, M. Photoelectrochemical studies on nanocrystalline hematite films. Chem. Mater. 6, 858–863 (1994).
Kay, A., Cesar, I. & Grätzel, M. New benchmark for water photooxidation by nanostructured α-Fe2O3 films. J. Am. Chem. Soc. 128, 15714–15721 (2006).
Jang, J.-W. et al. Enabling unassisted solar water splitting by iron oxide and silicon. Nature Commun. 6, 7447 (2015).
Zandi, O. & Hamann, T. Enhanced water splitting efficiency through selective surface state removal. J. Phys. Chem. Lett. 5, 1522–1526 (2014).
Sivula, K., Le Formal, F. & Grätzel, M. Solar water splitting: progress using haematite (α-Fe2O3) photoelectrodes. ChemSusChem 4, 432–449 (2011).
Hamann, T. W. Splitting water with rust: hematite photoelectrochemistry. Dalton Trans. 41, 7830–7834 (2012).
Kim, J. Y. et al. Single-crystalline, wormlike hematite photoanodes for efficient solar water splitting. Sci. Rep. 3, 1–8 (2013).
Warren, S. C. et al. Identifying champion nanostructures for solar water-splitting. Nature Mater. 12, 842–849 (2013).
Klahr, B. M. & Hamann, T. W. Voltage dependent photocurrent of thin film hematite electrodes. Appl. Phys. Lett. 99, 063508 (2011).
Klahr, B., Gimenez, S., Fabregat-Santiago, F., Hamann, T. & Bisquert, J. Water oxidation at hematite photoelectrodes: the role of surface states. J. Am. Chem. Soc. 134, 4294–4302 (2012).
Pendlebury, S. R. et al. Correlating long-lived photogenerated hole populations with photocurrent densities in hematite water oxidation photoanodes. Energy Environ. Sci. 5, 6304–6312 (2012).
Dotan, H., Sivula, K., Grätzel, M., Rothschild, A. & Warren, S. C. Probing the photoelectrochemical properties of hematite (α-Fe2O3) electrodes using hydrogen peroxide as a hole scavenger. Energy Environ. Sci. 4, 958–964 (2011).
Cummings, C. Y., Marken, F., Peter, L. M., Tahir, A. A. & Wijayantha, K. G. U. Kinetics and mechanism of light-driven oxygen evolution at thin film α-Fe2O3 electrodes. Chem. Commun. 48, 2027–2029 (2012).
Young, K. M. H., Klahr, B. M., Zandi, O. & Hamann, T. W. Photocatalytic water oxidation with hematite electrodes. Catal. Sci. Technol. 3, 1660–1671 (2013).
Upul Wijayantha, K. G., Saremi-Yarahmadi, S. & Peter, L. M. Kinetics of oxygen evolution at α-Fe2O3 photoanodes: a study by photoelectrochemical impedance spectroscopy. Phys. Chem. Chem. Phys. 13, 5264–5270 (2011).
Barroso, M., Pendlebury, S., Cowan, A. & Durrant, J. Charge carrier trapping, recombination and transfer in haematite (α-Fe2O3) water splitting photoanodes. Chem. Sci. 4, 2724–2734 (2013).
Cummings, C. Y., Marken, F., Peter, L. M., Upul Wijayantha, K. G. & Tahir, A. A. New insights into water splitting at mesoporous α-Fe2O3 films: a study by modulated transmittance and impedance spectroscopies. J. Am. Chem. Soc. 134, 1228–1234 (2012).
Barroso, M. et al. Dynamics of photogenerated holes in surface modified α-Fe2O3 photoanodes for solar water splitting. Proc. Natl Acad. Sci. USA 109, 15640–15645 (2012).
Klahr, B., Gimenez, S., Fabregat-Santiago, F., Bisquert, J. & Hamann, T. W. Electrochemical and photoelectrochemical investigation of water oxidation with hematite electrodes. Energy Environ. Sci. 5, 7626–7636 (2012).
Klahr, B. M., Gimenez, S., Zandi, O., Fabregat-Santiago, F. & Hamann, T. W. Competitive photoelectrochemical methanol and water oxidation with hematite electrodes. ACS Appl. Mater. Interfaces 7, 7653–7660 (2015).
Klahr, B. & Hamann, T. Water oxidation on hematite photoelectrodes: insight into the nature of surface states through in situ spectroelectrochemistry. J. Phys. Chem. C 118, 10393–10399 (2014).
Trainor, T. P. et al. Structure and reactivity of the hydrated hematite (0001) surface. Surf. Sci. 573, 204–224 (2004).
Hellman, A. & Pala, R. G. S. First principles study of photoinduced water-splitting on Fe2O3 . J. Phys. Chem. C 115, 12901–12907 (2011).
Yatom, N., Neufeld, O. & Toroker, M. C. Towards settling the debate on the role of Fe2O3 surface states for water splitting. J. Phys. Chem. C 119, 24789–24795 (2015).
Peter, L. M., Wijayantha, K. G. U. & Tahir, A. A. Kinetics of light-driven oxygen evolution at α-Fe2O3 electrodes. Discuss. Faraday Soc. 155, 309–322 (2012).
Machan, C. W., Sampson, M. D., Chabolla, S. A., Dang, T. & Kubiak, C. P. Developing a mechanistic understanding of molecular electrocatalysts for CO2 reduction using infrared spectroelectrochemistry. Organometallics 33, 4550–4559 (2014).
Araujo, P. Z. et al. FT-IR–ATR as a tool to probe photocatalytic interfaces. Colloids Surf. A 265, 73–80 (2005).
Bigi, J. P. et al. A high-spin iron(IV)–oxo complex supported by a trigonal nonheme pyrrolide platform. J. Am. Chem. Soc. 134, 1536–1542 (2012).
McDonald, A. R. & Que, L. High-valent nonheme iron–oxo complexes: synthesis, structure, and spectroscopy. Coord. Chem. Rev. 257, 414–428 (2013).
Shi, H., Lercher, J. A. & Yu, X.-Y. Sailing into uncharted waters: recent advances in the in situ monitoring of catalytic processes in aqueous environments. Catal. Sci. Technol. 5, 3035–3060 (2015).
Nakamura, R. & Nakato, Y. Primary intermediates of oxygen photoevolution reaction on TiO2 (rutile) particles, revealed by in situ FTIR absorption and photoluminescence measurements. J. Am. Chem. Soc. 126, 1290–1298 (2004).
Zhang, M., de Respinis, M. & Frei, H. Time-resolved observations of water oxidation intermediates on a cobalt oxide nanoparticle catalyst. Nature Chem. 6, 362–367 (2014).
Sivasankar, N., Weare, W. W. & Frei, H. Direct observation of a hydroperoxide surface intermediate upon visible light-driven water oxidation at an Ir oxide nanocluster catalyst by rapid-scan FT-IR spectroscopy. J. Am. Chem. Soc 133, 12976–12979 (2011).
Tiago de Oliveira, F. et al. Chemical and spectroscopic evidence for an FeV–oxo complex. Science 315, 835–838 (2007).
Green, M. T. Application of Badger's rule to heme and non-heme iron–oxygen bonds: an examination of ferryl protonation states. J. Am. Chem. Soc. 128, 1902–1906 (2006).
Bonnot, F. et al. Formation of high-valent iron–oxo species in superoxide reductase: characterization by resonance Raman spectroscopy. Angew. Chem. Int. Ed. 53, 5926–5930 (2014).
Nakamura, R., Imanishi, A., Murakoshi, K. & Nakato, Y. In situ FTIR studies of primary intermediates of photocatalytic reactions on nanocrystalline TiO2 films in contact with aqueous solutions. J. Am. Chem. Soc. 125, 7443–7450 (2003).
Proshlyakov, D. A., Henshaw, T. F., Monterosso, G. R., Ryle, M. J. & Hausinger, R. P. Direct detection of oxygen intermediates in the non-heme Fe enzyme taurine/α-ketoglutarate dioxygenase. J. Am. Chem. Soc. 126, 1022–1023 (2004).
Yamada, H. & Hurst, J. K. Resonance Raman, optical spectroscopic, and EPR characterization of the higher oxidation states of the water oxidation catalyst, cis,cis-[(bpy)2Ru(OH2)]2O4+. J. Am. Chem. Soc. 122, 5303–5311 (2000).
Nakato, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds (John Wiley & Sons, 1986).
Nakamura, R., Tanaka, T. & Nakato, Y. Oxygen photoevolution on a tantalum oxynitride photocatalyst under visible-light irradiation: how does water photooxidation proceed on a metal–oxynitride surface? J. Phys. Chem. B 109, 8920–8927 (2005).
Nakamoto, K. et al. Resonance Raman and infrared spectra of molecular oxygen adducts of N,N′-ethylenebis(2,2-diacetylethylideneaminato)cobalt(II). J. Am. Chem. Soc. 104, 3386–3391 (1982).
Banerjee, R., Proshlyakov, Y., Lipscomb, J. D. & Proshlyakov, D. A. Structure of the key species in the enzymatic oxidation of methane to methanol. Nature 518, 431–434 (2015).
Klahr, B. M., Martinson, A. B. F. & Hamann, T. W. Photoelectrochemical investigation of ultrathin film iron oxide solar cells prepared by atomic layer deposition. Langmuir 27, 461–468 (2011).
Acknowledgements
This work was supported by the National Science Foundation (CHE-1150378). The authors thank M. Bruening for the generous access to his infrared spectrometer.
Author information
Authors and Affiliations
Contributions
O.Z. and T.W.H. conceived and designed the experiments. O.Z. performed the experiments and analysed the data. O.Z. and T.W.H. co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 549 kb)
Rights and permissions
About this article
Cite this article
Zandi, O., Hamann, T. Determination of photoelectrochemical water oxidation intermediates on haematite electrode surfaces using operando infrared spectroscopy. Nature Chem 8, 778–783 (2016). https://doi.org/10.1038/nchem.2557
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2557
This article is cited by
-
Br−/BrO−-mediated highly efficient photoelectrochemical epoxidation of alkenes on α-Fe2O3
Nature Communications (2023)
-
Catalytic Mechanisms and Active Species of Benzene Hydroxylation Reaction System Based on Fe-Based Enzyme-Mimetic Structure
Catalysis Letters (2023)
-
Inductive effect of Ti-doping in Fe2O3 enhances the photoelectrochemical water oxidation
Science China Chemistry (2023)
-
Boosting multi-hole water oxidation catalysis on hematite photoanodes under low bias
Science China Chemistry (2023)
-
Atomic regulations of single atom from metal-organic framework derived carbon for advanced water treatment
Nano Research (2023)