Abstract
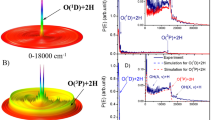
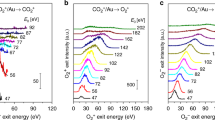
Until recently, it was widely regarded that only one reaction pathway led to the production of molecular oxygen in Earth's prebiotic primitive atmosphere: a three-body recombination reaction of two oxygen atoms and a third body that removes excess energy. However, an additional pathway has recently been observed that involves the photodissociation of CO2 on exposure to ultraviolet light. Here we demonstrate a further pathway to O2 production, again from CO2, but via dissociative electron attachment (DEA). Using anion-velocity image mapping, we provide experimental evidence for a channel of DEA to CO2 that produces O2(X3Σ−g) + C−. This observed channel coexists in the same energy range as the competitive three-body dissociation of CO2 to give O + O + C−. The abundance of low-energy electrons in interstellar space and the upper atmosphere of Earth suggests that the contributions of these pathways are significant and should be incorporated into atmospheric chemistry models.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Holland, H. D. The oxygenation of the atmosphere and oceans. Phil. Trans. R. Soc. B 361, 903–915 (2006).
Kasting, J. F., Pollack, J. B. & Crisp, D. Effects of high CO2 levels on surface-temperature and atmospheric oxidation state of the early Earth. J. Atmos. Chem. 1, 403–428 (1984).
Kasting, J. F. & Walker, J. C. Limits on oxygen concentration in the prebiological atmosphere and the rate of abiotic fixation of nitrogen. J. Geophys. Res. 86, 1147–1158 (1981).
Kasting, J. F., Liu, S. C. & Donahue, T. M. Oxygen levels in the prebiological atmosphere. J. Geophys. Res. 84, 3097–3107 (1979).
Meriwether, J. W. Jr. A review of the photochemistry of selected nightglow emissions from the mesopause. J. Geophys. Res. 94, 14629–14646 (1989).
Slanger, T. G. & Copeland, R. A. Energetic oxygen in the upper atmosphere and the laboratory. Chem. Rev. 103, 4731–4765 (2003).
Pejakvović, D. A., Copeland, R. A., Cosby, P. C. & Slanger, T. G. Studies on the production of O2(a1Δg, ν = 0) and O2(b1Σg+, ν = 0) from collisional removal of O2(A3Σu+, ν = 6–10). J. Geophys. Res. 112, A10307 (2007).
Lu, Z., Chang, Y. C., Yin, Q.-Z., Ng, C. Y. & Jackson, W. M. Evidence for direct molecular oxygen production in CO2 photodissociation. Science 346, 61–64 (2014).
Suits, A. G. & Parker, D. H. Hot molecules—off the beaten path. Science 346, 30–31 (2014).
Lee, J. S., Doering, J. P., Potemra, T. A. & Brace, L. H. Measurements of the ambient photoelectron spectrum from atmosphere explorer: I. AE-E measurements below 300 km during solar minimum conditions. Planet. Space Sci. 28, 947–971 (1980).
Lee, J. S., Doering, J. P., Potemra, T. A. & Brace, L. H. Measurements of the ambient photoelectron spectrum from atmosphere explorer: II. AE-E measurements from 300 to 1000 km during solar minimum conditions. Planet. Space Sci. 28, 973–996 (1980).
Christophorou, L. G. Electron Molecule Interactions and their Applications (Academic Press, 1984).
Chantry, P. J. Dissociative attachment in carbon dioxide. J. Chem. Phys. 57, 3180–3186 (1972).
McCurdy, C. W., Isaacs, W. A., Meyer, H.-D. & Rescigno, T. N. Resonant vibrational excitation of CO2 by electron impact: nuclear dynamics on the coupled components of the 2Πu resonance. Phys. Rev. A 67, 042708 (2003).
Claydon, C. R., Segal, G. A. & Taylor, H. S. Theoretical interpretation of the electron scattering spectrum of CO2 . J. Chem. Phys. 52, 3387–3398 (1970).
Spence, D. & Schulz, G. J. Cross sections for production of O2− and C− by dissociative electron attachment in CO2: an observation of the Renner–Teller effect. J. Chem. Phys. 60, 216–220 (1974).
Wu, B. et al. Renner–Teller effect on dissociative electron attachments to carbon dioxide. Phys. Rev. A 85, 052709 (2012).
Moradmand, A. et al. Dissociative electron attachment to carbon dioxide via the 2Πu shape resonance. Phys. Rev. A 88, 032703 (2013).
Wu, B., Xia, L., Li, H.-K., Zeng, X.-J. & Tian, S. X. Positive/negative ion velocity mapping apparatus for electron–molecule reactions. Rev. Sci. Instrum. 83, 013108 (2012).
Wu, C. et al. Nonsequential and sequential fragmentation of CO23+ in intense laser fields. Phys. Rev. Lett. 110, 103601 (2013).
Krupenie, P. The spectrum of molecular oxygen. J. Phys. Chem. Ref. Data 1, 423–534 (1972).
Allan, M. et al. Production of vibrationally autodetaching O− in low-energy electron impact on ozone. J. Phys. B 29, 3487–3495 (1996).
O'Malley, T. F. & Taylor, H. S. Angular dependence of scattering products in electron–molecule resonant excitation and in dissociative attachment. Phys. Rev. 176, 207–221 (1968).
Klar, H. & Morgner, H. Angular distribution of negative ions formed in dissociative attachment. J. Phys. B 12, 2369–2377 (1979).
Chapman, S. Some phenomena of the upper atmosphere. Proc. R. Soc. Lond. A 132, 353–374 (1931).
Xia, L. et al. Communication: state mixing by spin–orbit coupling in the anionic chloroiodine dissociations. J. Chem. Phys. 140, 041106 (2014).
Acknowledgements
This work is supported by Natural Science Foundation of China (Grant No. 21273213), the Ministry of Science and Technology of China (Grant No. 2013CB834602) and Hefei Science Center of Chinese Academy of Sciences.
Author information
Authors and Affiliations
Contributions
S.X.T. supervised the study. X.-D.W., X.-F.G. and C.-J.X. carried out the experimental measurements, and X.-D.W., X-.F.G. and S.X.T. performed the data analysis. X.-D.W. and S.X.T. wrote the paper. All the authors discussed the results and contributed to the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 387 kb)
Rights and permissions
About this article
Cite this article
Wang, XD., Gao, XF., Xuan, CJ. et al. Dissociative electron attachment to CO2 produces molecular oxygen. Nature Chem 8, 258–263 (2016). https://doi.org/10.1038/nchem.2427
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2427