Abstract
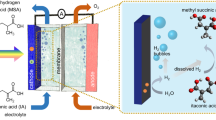
In a typical hydrogen-producing photoelectrochemical cell (PEC), water reduction at the cathode (producing hydrogen) is accompanied by water oxidation at the anode (producing oxygen). This anode reaction is, however, not kinetically favourable. Here we investigate the possibility of utilizing solar energy for biomass conversion by performing the oxidation of 5-hydroxymethylfurfural (HMF) into 2,5-furandicarboxylic acid (FDCA) at the anode of a PEC. HMF is a key intermediate in biomass conversion, and FDCA is an important monomer for the production of numerous polymers. Using 2,2,6,6-tetramethylpiperidine-1-oxyl as a mediator, we obtained a near-quantitative yield and 100% Faradaic efficiency at ambient conditions without the use of precious-metal catalysts. This reaction is also thermodynamically and kinetically more favourable than water oxidation. Our results suggest that solar-driven biomass conversion can be a viable anode reaction that has the potential to increase both the efficiency and the utility of PECs constructed for solar-fuel production.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wrighton, M. S. Photoelectrochemical conversion of optical energy to electricity and fuels. Acc. Chem. Res. 12, 303–310 (1979).
Lewis, N. S. & Nocera, D. G. Powering the planet: chemical challenges in solar energy utilization. Proc. Natl Acad. Sci. USA 103, 15729–15735 (2006).
Walter, M. G. et al. Solar water splitting cells. Chem. Rev. 110, 6446–6473 (2010).
Kumar, B. et al. Photochemical and photoelectrochemical reduction of CO2 . Annu. Rev. Phys. Chem. 63, 541–569 (2012).
Centi, G. & Perathoner, S. Opportunities and prospects in the chemical recycling of carbon dioxide to fuels. Catal. Today 148, 191–205 (2009).
Hu, L. et al. Catalytic conversion of biomass-derived carbohydrates into fuels and chemicals via furanic aldehydes. RSC Adv. 2, 11184–11206 (2012).
Corma, A., Iborra, S. & Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 107, 2411–2502 (2007).
Tong, X., Ma, Y. & Li, Y. Biomass into chemicals: conversion of sugars to furan derivatives by catalytic processes. Appl. Catal. A 385, 1–13 (2010).
Rosatella, A. A., Simeonov, S. P., Frade, R. F. & Afonso, C. A. 5-Hydroxymethylfurfural (HMF) as a building block platform: biological properties, synthesis and synthetic applications. Green Chem. 13, 754–793 (2011).
Wang, T., Nolte, M. W. & Shanks, B. H. Catalytic dehydration of C6 carbohydrates for the production of hydroxymethylfurfural (HMF) as a versatile platform chemical. Green Chem. 16, 548–572 (2014).
Bozell, J. J. & Petersen, G. R. Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy's ‘top 10’ revisited. Green Chem. 12, 539–554 (2010).
Gandini, A., Silvestre, A. J., Neto, C. P., Sousa, A. F. & Gomes, M. The furan counterpart of poly (ethylene terephthalate): an alternative material based on renewable resources. J. Polym. Sci. A 47, 295–298 (2009).
Verdeguer, P., Merat, N. & Gaset, A. Oxydation catalytique du HMF en acide 2,5-furane dicarboxylique. J. Mol. Catal. 85, 327–344 (1993).
Casanova, O., Iborra, S. & Corma, A. Biomass into chemicals: aerobic oxidation of 5-hydroxymethyl-2-furfural into 2,5-furandicarboxylic acid with gold nanoparticle catalysts. ChemSusChem 2, 1138–1144 (2009).
Gorbanev, Y. Y., Klitgaard, S. K., Woodley, J. M., Christensen, C. H. & Riisager, A. Gold-catalyzed aerobic oxidation of 5-hydroxymethylfurfural in water at ambient temperature. ChemSusChem 2, 672–675 (2009).
Villa, A., Schiavoni, M., Campisi, S., Veith, G. M. & Prati, L. Pd-modified Au on carbon as an effective and durable catalyst for the direct oxidation of HMF to 2,5-furandicarboxylic acid. ChemSusChem 6, 609–612 (2013).
Taarning, E., Nielsen, I. S., Egeblad, K., Madsen, R. & Christensen, C. H. Chemicals from renewables: aerobic oxidation of furfural and hydroxymethylfurfural over gold catalysts. ChemSusChem 1, 75–78 (2008).
Davis, S. E., Houk, L. R., Tamargo, E. C., Datye, A. K. & Davis, R. J. Oxidation of 5-hydroxymethylfurfural over supported Pt, Pd and Au catalysts. Catal. Today 160, 55–60 (2011).
Pasini, T. et al. Selective oxidation of 5-hydroxymethyl-2-furfural using supported gold–copper nanoparticles. Green Chem. 13, 2091–2099 (2011).
Grabowski, G., Lewkowski, J. & Skowroński, R. The electrochemical oxidation of 5-hydroxymethylfurfural with the nickel oxide/hydroxide electrode. Electrochim. Acta 36, 1995 (1991).
Skowroski, R., Cottier, L., Descotes, G. & Lewkowski, J. Selective anodic oxidation of 5-hydroxymethylfurfural. Synthesis 1996, 1291–1292 (1996).
Vuyyuru, K. R. & Strasser, P. Oxidation of biomass derived 5-hydroxymethylfurfural using heterogeneous and electrochemical catalysis. Catal. Today 195, 144–154 (2012).
Chadderdon, D. J. et al. Electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid on supported Au and Pd bimetallic nanoparticles. Green Chem. 16, 3778–3786 (2014).
Schnatbaum, K. & Schäfer, H. J. Electroorganic synthesis 66: selective anodic oxidation of carbohydrates mediated by TEMPO. Synthesis 1999, 864–872 (1999).
Dijkman, W. P., Groothuis, D. E. & Fraaije, M. W. Enzyme-catalyzed oxidation of 5-hydroxymethylfurfural to furan-2,5-dicarboxylic acid. Angew. Chem. Int. Ed. 53, 6515–6518 (2014).
Davis, S. E., Zope, B. N. & Davis, R. J. On the mechanism of selective oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over supported Pt and Au catalysts. Green Chem. 14, 143–147 (2012).
Kim, T. W. & Choi, K-S. Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science 343, 990–994 (2014).
Park, Y., McDonald, K. J. & Choi, K-S. Progress in bismuth vanadate photoanodes for use in solar water oxidation. Chem. Soc. Rev. 42, 2321–2337 (2013).
Acknowledgements
This work was supported by the University of Wisconsin-Madison, the Camille and Henry Dreyfus Postdoctoral Program in Environmental Chemistry and the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the US Department of Energy through Grant DE-SC0008707. The authors thank L. M. Smith and Q. Li for the use of the HPLC.
Author information
Authors and Affiliations
Contributions
K-S.C. supervised the project. H.G.C. carried out all the experiments. H.G.C. and K-S.C. analysed the results and prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1182 kb)
Rights and permissions
About this article
Cite this article
Cha, H., Choi, KS. Combined biomass valorization and hydrogen production in a photoelectrochemical cell. Nature Chem 7, 328–333 (2015). https://doi.org/10.1038/nchem.2194
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2194
This article is cited by
-
Techno-economic Analysis and Life Cycle Impact Assessment for the Valorisation of Kraft Lignin and Low-Voltage Hydrogen Production
Korean Journal of Chemical Engineering (2024)
-
Approaches to Improving Selectivity During Photoelectrochemical Transformation of Small Molecules
Transactions of Tianjin University (2024)
-
Selective photoelectrochemical oxidation of glucose to glucaric acid by single atom Pt decorated defective TiO2
Nature Communications (2023)
-
Scalable electrosynthesis of commodity chemicals from biomass by suppressing non-Faradaic transformations
Nature Communications (2023)
-
Renewable formate from sunlight, biomass and carbon dioxide in a photoelectrochemical cell
Nature Communications (2023)