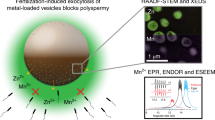
Abstract
Fertilization of a mammalian egg initiates a series of ‘zinc sparks’ that are necessary to induce the egg-to-embryo transition. Despite the importance of these zinc-efflux events little is known about their origin. To understand the molecular mechanism of the zinc spark we combined four physical approaches that resolve zinc distributions in single cells: a chemical probe for dynamic live-cell fluorescence imaging and a combination of scanning transmission electron microscopy with energy-dispersive spectroscopy, X-ray fluorescence microscopy and three-dimensional elemental tomography for high-resolution elemental mapping. We show that the zinc spark arises from a system of thousands of zinc-loaded vesicles, each of which contains, on average, 106 zinc atoms. These vesicles undergo dynamic movement during oocyte maturation and exocytosis at the time of fertilization. The discovery of these vesicles and the demonstration that zinc sparks originate from them provides a quantitative framework for understanding how zinc fluxes regulate cellular processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
23 December 2014
In the version of this Article previously published online, the NIH grant number T32GM105538 was missing from the Acknowledgements section. This has now been corrected in all versions of the Article.
References
Berg, J. M. & Shi, Y. The galvanization of biology: a growing appreciation for the roles of zinc. Science 271, 1081–1085 (1996).
Finney, L. A. & O'Halloran, T. V. Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science 300, 931–936 (2003).
O'Halloran, T. V. Transition metals in control of gene expression. Science 261, 715–725 (1993).
Maret, W. Zinc biochemistry: from a single zinc enzyme to a key element of life. Adv. Nutr. 4, 82–91 (2013).
Kim, A. M., Vogt, S., O'Halloran, T. V. & Woodruff, T. K. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nature Chem. Biol. 6, 674–681 (2010).
Kim, A. M. et al. Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs. ACS Chem. Biol. 6, 716–723 (2011).
Bernhardt, M. L., Kim, A. M., O'Halloran, T. V. & Woodruff, T. K. Zinc requirement during meiosis I–meiosis II transition in mouse oocytes is independent of the MOS–MAPK pathway. Biol. Reprod. 84, 526–536 (2011).
Bernhardt, M. L., Kong, B. Y., Kim, A. M., O'Halloran, T. V. & Woodruff, T. K. A zinc-dependent mechanism regulates meiotic progression in mammalian oocytes. Biol. Reprod. 86, 114 (2012).
Kong, B. Y., Bernhardt, M. L., Kim, A. M., O'Halloran, T. V. & Woodruff, T. K. Zinc maintains prophase I arrest in mouse oocytes through regulation of the MOS–MAPK pathway. Biol. Reprod. 87, 11, 11–12 (2012).
Suzuki, T., Yoshida, N., Suzuki, E., Okuda, E. & Perry, A. C. Full-term mouse development by abolishing Zn2+-dependent metaphase II arrest without Ca2+ release. Development 137, 2659–2669 (2010).
Tian, X. & Diaz, F. J. Zinc depletion causes multiple defects in ovarian function during the periovulatory period in mice. Endocrinology 153, 873–886 (2012).
Krauchunas, A. R. & Wolfner, M. F. in Gametogenesis (ed. Wassarman P. M.) 267–292 (Current Topics in Developmental Biology, 102, Academic Press, 2013).
Suzuki, T. et al. Mouse Emi2 as a distinctive regulatory hub in second meiotic metaphase. Development 137, 3281–3291 (2010).
Kong, B. Y. et al. Maternally-derived zinc transporters ZIP6 and ZIP10 drive the mammalian oocyte-to-egg transition. Mol. Hum. Reprod. 20, 1077–1089 (2014).
Catterall, A. Structure and function of voltage-gated ion channels. Annu. Rev. Biochem. 64, 493–531 (1995).
Cousins, R. J., Liuzzi, J. P. & Lichten, L. A. Mammalian zinc transport, trafficking, and signals. J. Biol. Chem. 281, 24085–24089 (2006).
Kühlbrandt, W. Biology, structure and mechanism of P-type ATPases. Nature Rev. Mol. Cell Biol. 5, 282–295 (2004).
Chimienti, F., Devergnas, S., Favier, A. & Seve, M. Identification and cloning of a beta-cell-specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes 53, 2330–2337 (2004).
Frederickson, C. J., Suh, S. W., Silva, D., Frederickson, C. J. & Thompson, R. B. Importance of zinc in the central nervous system: the zinc-containing neuron. J. Nutr. 130, 1471S–1483S (2000).
Palmiter, R. D., Cole, T. B., Quaife, C. J. & Findley, S. D. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc. Natl Acad. Sci. USA 93, 14934–14939 (1996).
Yamasaki, S. et al. Zinc is a novel intracellular second messenger. J. Cell Biol. 177, 637–645 (2007).
Zalewski, P. D. et al. Video image analysis of labile zinc in viable pancreatic islet cells using a specific fluorescent probe for zinc. J. Histochem. Cytochem. 42, 877–884 (1994).
Fierke, C. A. & Thompson, R. B. Fluorescence-based biosensing of zinc using carbonic anhydrase. Biometals 14, 205–222 (2001).
Palmer, A. E., Qin, Y., Park, J. G. & McCombs, J. E. Design and application of genetically encoded biosensors. Trends Biotechnol. 29, 144–152 (2011).
Que, E. L., Domaille, D. W. & Chang, C. J. Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem. Rev. 108, 1517–1549 (2008).
Tomat, E. & Lippard, S. J. Imaging mobile zinc in biology. Curr. Opin. Chem. Biol. 14, 225–230 (2010).
Fahrni, C. J. & O'Halloran, T. V. Aqueous coordination chemistry of quinoline-based fluorescence probes for the biological chemistry of zinc. J. Am. Chem. Soc. 121, 11448–11458 (1999).
Cork, R. Problems with the application of quin-2-AM to measuring cytoplasmic free calcium in plant cells. Plant Cell Environ. 9, 157–161 (1986).
Laha, J. K., Dhanalekshmi, S., Taniguchi, M., Ambroise, A. & Lindsey, J. S. A Scalable synthesis of meso-substituted dipyrromethanes. Org. Proc. Res. Devel. 7, 799–812 (2003).
Domaille, D. W., Zeng, L. & Chang, C. J. Visualizing ascorbate-triggered release of labile copper within living cells using a ratiometric fluorescent sensor. J. Am. Chem. Soc. 132, 1194–1195 (2010).
Hureau, C. et al. Syntheses, X-ray structures, solid state high-field electron paramagnetic resonance, and density-functional theory investigations on chloro and aqua MnII mononuclear complexes with amino-pyridine pentadentate ligands. Inorg. Chem. 47, 9238–9247 (2008).
Ambundo, E. A. et al. Influence of coordination geometry upon copper(II/I) redox potentials. Physical parameters for twelve copper tripodal ligand complexes. Inorg. Chem. 38, 4233–4242 (1999).
Rae, T. D., Schmidt, P. J., Pufahl, R. A., Culotta, V. C. & O'Halloran, T. V. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science 284, 805–808 (1999).
Andrews, J. C., Nolan, J. P., Hammerstedt, R. H. & Bavister, B. D. Role of zinc during hamster sperm capacitation. Biol. Reprod. 51, 1238–1247 (1994).
Stoltenberg, M. et al. Autometallographic demonstration of zinc ions in rat sperm cells. Mol. Hum. Reprod. 3, 763–767 (1997).
Zalewski, P. et al. Use of a zinc fluorophore to measure labile pools of zinc in body fluids and cell-conditioned media. Biotechniques 40, 509–520 (2006).
Outten, C. E. & O'Halloran, T. V. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 292, 2488–2492 (2001).
Fahrni, C. J. Synthetic fluorescent probes for monovalent copper. Curr. Opin. Chem. Biol. (2013).
Gee, K. R., Zhou, Z. L., Qian, W. J. & Kennedy, R. Detection and imaging of zinc secretion from pancreatic beta-cells using a new fluorescent zinc indicator. J. Am. Chem. Soc. 124, 776–778 (2002).
Burdette, S. C., Frederickson, C. J., Bu, W. & Lippard, S. J. ZP4, an improved neuronal Zn2+ sensor of the Zinpyr family. J. Am. Chem. Soc. 125, 1778–1787 (2003).
Ducibella, T., Anderson, E., Albertini, D. F., Aalberg, J. & Rangarajan, S. Quantitative studies of changes in cortical granule number and distribution in the mouse oocyte during meiotic maturation. Dev. Biol. 130, 184–197 (1988).
Wessel, G. M. et al. The biology of cortical granules. Int. Rev. Cytol. 209, 117–206 (2001).
Burkart, A. D., Xiong, B., Baibakov, B., Jimenez-Movilla, M. & Dean, J. Ovastacin, a cortical granule protease, cleaves ZP2 in the zona pellucida to prevent polyspermy. J. Cell Biol. 197, 37–44 (2012).
Tahara, M. et al. Dynamics of cortical granule exocytosis at fertilization in living mouse eggs. Am. J. Physiol. 270, C1354–1361 (1996).
Stika, K. M., Bielat, K. L. & Morrison, G. H. Diffusible ion localization by ion microscopy: a comparison of chemically prepared and fast-frozen, freeze-dried, unfixed liver sections. J. Microsc. 118, 409–420 (1980).
Timm, F. Histochemistry of heavy metals; the sulfide–silver procedure. Dtsch Z. Gesamte Gerichtl Med. 46, 706–711 (1958).
Danscher, G., Stoltenberg, M., Bruhn, M., Sondergaard, C. & Jensen, D. Immersion autometallography: histochemical in situ capturing of zinc ions in catalytic zinc–sulfur nanocrystals. J. Histochem. Cytochem. 52, 1619–1625 (2004).
Licht, S. Aqueous solubilities, solubility products and standard oxidation–reduction potentials of the metal sulfides. J. Electrochem. Soc. 135, 2971–2975 (1988).
Wu, J. S. et al. Imaging and elemental mapping of biological specimens with a dual-EDS dedicated scanning transmission electron microscope. Ultramicroscopy 128, 24–31 (2013).
Chen, S. et al. The Bionanoprobe: hard X-ray fluorescence nanoprobe with cryogenic capabilities. J. Synchrotron Radiat. 21, 66–75 (2014).
Hong, Y. P. et al. Alignment of low-dose X-ray fluorescence tomography images using differential phase contrast. J. Synchrotron Radiat. 21, 229–234 (2014).
Gleber, S-C. et al. New developments in hard X-ray fluorescence microscopy for in-situ investigations of trace element distributions in aqueous systems of soil colloids. J. Phys. Conf. Ser. 463, 012005 (2013).
Frederickson, C. J., Klitenick, M. A., Manton, W. I. & Kirkpatrick, J. B. Cytoarchitectonic distribution of zinc in the hippocampus of man and the rat. Brain Res. 273, 335–339 (1983).
Foresta, C. et al. Role of zinc trafficking in male fertility: from germ to sperm. Hum. Reprod. 29, 1134–1145 (2014).
Lishko, P. V. & Kirichok, Y. The role of Hv1 and CatSper channels in sperm activation. J. Physiol. 588, 4667–4672 (2010).
Acknowledgements
The authors thank members of the O'Halloran, Woodruff and Dravid labs for scientific discussions and advice. We thank E. W. Roth for the preparation of electron microscopy samples and J-H. Chung and J. Shangguan for help with chemical syntheses. Equipment and experimental guidance were provided by the following core facilities at Northwestern University: the Integrated Molecular Structure Education and Research Center, the Biological Imaging Facility, the Quantitative Bioelemental Imaging Center, the Electron Probe Instrumentation Centre and the Keck Biophysics Facility. This research used resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. This work was supported by a Medical Research Award from the W. M. Keck Foundation, a SPARK Award from the Chicago Biomedical Consortium and the National Institutes of Health (P01 HD021921, GM38784, U54HD076188 and T32GM105538).
Author information
Authors and Affiliations
Contributions
E.L.Q., R.B., F.E.D., B.Y.K., V.P.D., T.K.W. and T.V.O. designed the research. E.L.Q., R.B., F.E.D., B.Y.K., S.A.G. and A.R.B. performed the research. S.C.G., S.V. and S.C. helped design and implement XFM experiments and process and analyse the data. E.L.Q., R.B., F.E.D., T.K.W. and T.V.O. wrote the manuscript. All the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 6382 kb)
Supplementary movie 1
Supplementary movie 1 (MOV 822 kb)
Supplementary movie 2
Supplementary movie 2 (MOV 12334 kb)
Supplementary movie 3
Supplementary movie 3 (MOV 5802 kb)
Rights and permissions
About this article
Cite this article
Que, E., Bleher, R., Duncan, F. et al. Quantitative mapping of zinc fluxes in the mammalian egg reveals the origin of fertilization-induced zinc sparks. Nature Chem 7, 130–139 (2015). https://doi.org/10.1038/nchem.2133
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2133
This article is cited by
-
The role of zinc in follicular development
Molecular Biology Reports (2023)
-
The Implications of Insufficient Zinc on the Generation of Oxidative Stress Leading to Decreased Oocyte Quality
Reproductive Sciences (2023)
-
Single molecule microscopy to profile the effect of zinc status on transcription factor dynamics
Scientific Reports (2022)
-
Zinc is a master-regulator of sperm function associated with binding, motility, and metabolic modulation during porcine sperm capacitation
Communications Biology (2022)
-
Transglutaminase 2 crosslinks zona pellucida glycoprotein 3 to prevent polyspermy
Cell Death & Differentiation (2022)